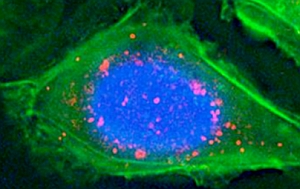
Fluorescence microscopy image of nanoparticle accumulations, in pink, on cell surface antigen (BIND Biosciences)
Researchers from four universities, three hospitals, and the biopharmaceutical company BIND Biosciences are testing a new form of cancer therapy using nanoscale particles designed to deliver a dose of targeted medicine to solid tumors. The findings, including results of early clinical trials, are published in this week’s issue of the journal Science Translational Medicine (paid subscription required).
For many cancer patients, drug dosages to treat the disease need to be moderated to prevent debilitating side effects from the drugs’ toxicity. BIND Biosciences, in Cambridge, Massachusetts, developed a technology based on research at MIT and Harvard Medical School to deliver doses of cancer-killing drugs more precisely to the tumor cells. Two of the study’s authors — Robert Langer of MIT and Omid Farokhzad of Harvard Medical School — are founders of BIND Biosciences, joined as authors by colleagues from BIND, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Translational Genomics Research Institute, Wayne State University, and Weill Cornell Medical College.
The studies test BIND-014, a targeted therapeutic nanoparticle with programmable pharmacological properties, including particle circulation time, pharmacokinetic (interactions with body chemistry) profile, biodistribution, and release properties. BIND-014 contains docetaxel, a drug approved to treat cancer of the breast, prostate and lung, encapsulated in FDA-approved biocompatible and biodegradable polymers. BIND-014 is targeted to prostate specific membrane antigen (PSMA), a cell surface antigen found on the surface of cancer cells and on new blood vessels that feed many solid tumors.
Lab tests of BIND-014 show pharmacokinetic characteristics indicating prolonged circulation and controlled drug release with plasma concentrations remaining at least 100 times higher than conventional docetaxel for over 24 hours. The lab tests also show a ten-fold increase in drug concentrations inside the tumors with more suppression of tumor growth in several types of tumor compared with conventional docetaxel.
BIND Biosciences has begun as well a phase 1 clinical trial to test the safety of BIND-014 and identify safe dosage levels of its treatments. In the trial, BIND-014 has so far been given to 17 heavily pretreated patients with advanced or metastatic solid tumor cancers. The company reports BIND-014 has been administered in dosages up to 75 milligrams per square meter of body surface area, with dose escalations in progress. To date, BIND-014 is generally well tolerated with no new toxicities observed.
While the main objective of the trial is drug safety, the company reports that in patients receiving BIND-014 the drug appears to work in similar ways as in the lab tests. The cancer-killing drug docetaxel accumulated in tumors, with tumor shrinkage occurring with doses as low as 20 percent of the normal prescribed docetaxel dose, and in cancers in which docetaxel usually has minimal effect.
“Previous attempts to develop targeted nanoparticles have not translated into clinical success,” notes MIT’s Langer, “because of the inherent difficulty of designing and scaling up a particle capable of targeting, long-circulation via immune-response evasion, and controlled drug release.” Harvard’s Farokhzad adds,”BIND-014, demonstrates for the first time that it is possible to generate medicines with both targeted and programmable properties that can concentrate the therapeutic effect directly at the site of disease, potentially revolutionizing how complex diseases such as cancer are treated.”
Read more:
- Medical Center Commercializes Nanotech Cancer Treatment
- Nanotech Helps Reduce Cancer Drug Side Effects
* * *

 RSS - Posts
RSS - Posts
[…] Programmed Nanoparticles Tested as Cancer Treatments […]