Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on September 6th, 2023  Antibody illustration (J.S. Choi, https://jschoi.org/20/body-emoji/) 6 Sept. 2023. A company formed by M.D. Anderson Cancer Center and Panacea Venture plans to develop synthetic antibodies as treatments addressing new cancer targets. Initial funding amounts from Panacea Ventures or others for Manaolana Oncology Inc. in Thousand Oaks, California were not disclosed.
Manaolana Oncology seeks to commercialize discoveries by researchers at M.D. Anderson Cancer Center in Houston, part of the University of Texas system. The company expects to develop monoclonal antibodies, synthetic proteins acting like natural antibodies in the immune system that attack specific protein targets called antigens unique to cancer cells. M.D. Anderson and Panacea Venture say the new biotechnology company’s output will address multiple types of cancer, designing candidate therapies for eventual clinical testing at M.D. Anderson.
One of the labs behind the work of Manaolana Oncology is headed by Samir Hanash, director of M.D. Anderson’s proteomics platform that studies proteins used by tumors to grow and develop. Hanash and colleagues investigate these proteins for unique features that offer targets useful for diagnostic tests and treatments, including immunotherapies that invoke an immune system response. Their work has led to a catalog of characteristic cancer proteins found on the surface of tumor cells providing targets across multiple cancer types. “By combing through petabytes of proteomic data,” says Hanash in a statement, “our team has uncovered many cancer-restricted antigens that may offer prime targets for monoclonal antibody therapies.”
Faster antibody design and production
Another M.D. Anderson lab with intellectual property for Manaolana Oncology is led by molecular pathologist Kevin McBride that studies basic functions of B lymphocytes, white blood cells in the immune system for producing antibodies. McBride and colleagues investigate the biology of B cells at fine genetic levels to better understand their affinity for antigens and binding behavior.
Their work resulted in processes for quickly designing and producing synthetic antibodies addressing specific cancer targets, instituted in M.D. Anderson’s Recombinant Antibody Production Core that generates engineered antibodies for research and treatments. “This is an important evolution,” notes McBride, “because current approaches to creating high-quality monoclonal antibodies require lengthy and intensive production protocols, which can be costly and are not always successful.”
M.D. Anderson and Panacea Venture say Manaolana Oncology expects to further refine these advances into new cancer treatments developed at a faster pace than before. “By developing therapies targeting new tumor antigens,” says company co-founder and CEO Winson Tang, “Manaolana Oncology aims to address a critical need for patients with cancer. Manaolana is the Hawaiian word for hope, and it is our intent to offer patients renewed hope against this disease.”
Manaolana Oncology is organizing at Panacea Venture, with Panacea funding start-up expenses and recruiting more company executives. Panacea Venture is a six year-old life science venture investor, based in the U.S. and China, supporting start-up enterprises from incubation through expansion. Tang, Manaolana Oncology’s CEO, is a Panacea venture partner.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 5th, 2023  (Semevent, Pixabay. https://pixabay.com/photos/examination-stethoscope-to-listen-2458540/) 5 Sept. 2023. Results of a clinical trial show a vaccine delivered as a nasal spray protects healthy people against infection from the bacteria causing whooping cough infections. The biotechnology company ILiAD Biotechnologies LLC, developer of the vaccine code-named BPZE1, announced findings from the challenge study, which are not yet peer-reviewed, in a statement today.
Whooping cough is a highly contagious respiratory disease, caused by infections from the Bordetella pertussis bacteria. The disease, also known as pertussis, results from the bacteria attaching to tiny hairs called cilia that line the upper respiratory organs, and releasing toxins that damage the lining of lungs and airways. Whooping cough spreads from person to person easily through coughs or sneezes carrying the bacteria. Infants infected with whooping cough can develop violent repeated coughing fits that last as long as 10 weeks.
Vaccines are available and recommended for young children, as well as preventive antibiotics for people exposed to the bacteria. As a result, according to Centers for Disease Control and Prevention, occurrence of whooping cough has largely receded in the U.S. since the 1920s, except for a brief recent period when some parents skipped vaccinations for their children. Nonetheless, children under one year remain those most likely to become infected with whooping cough.
Immune response in the nasal passages
ILiAD Biotechnologies in Weston, Florida develops vaccines and treatments for respiratory diseases, beginning with whooping cough. BPZE1 is the company’s lead product, designed as a live but weakened form of Bordetella pertussis bacteria, administered as a nasal spray to invoke an immune response in the nasal passages, where humans first encounter the bacteria. ILiAD Bio says its preclinical studies show one dose of BPZE1 works quickly to stimulate antibodies and T-cells that clear the bacteria and prevent disease, with four early- and mid-stage clinical trials inducing immune responses in humans without serious vaccine-related adverse effects. The company says its vaccine is aimed for adolescents and adults.
The recent clinical trial is a mid-stage study enrolling 53 healthy adults at two sites in the U.K. Participants were randomly assigned to receive a single nasal spray dose of BPZE1 or a placebo, then two to four months later exposed to enough Bordetella pertussis bacteria to cause disease. After that exposure, participants were quarantined at a clinic for 17 days, and given antibiotics if whooping cough symptoms developed, with antibiotics also given to those participants not developing symptoms at the end of the monitoring period. The study team from University of Oxford and University Hospital Southampton followed up with participants three and six months later.
ILiAD Bio says results show participants receiving BPZE1 with 98 percent less bacteria burden, measured as bacterial colony forming units or CFUs, than placebo recipients. In addition, BPZE1 recipients develop more antibodies in their mucous membranes and blood serum than placebo recipients. The company says BPZE1 was well tolerated, with mild symptom reactions of short duration, comparable to placebo recipients, and no serious adverse effects.
“The dramatic reduction in quantitative CFU counts in BPZE1 vaccinated participants compared to placebo,” says ILiAD Bio chief medical officer Stephanie Noviello in a company statement released through BusinessWire, “over the duration of post-challenge days, reinforces the potential clinical benefit of BPZE1, confirming previously published BPZE1 results in preclinical and attenuated human challenge studies.” Noviello adds that the company plans to present the findings to the World Vaccine Congress in Barcelona, Spain next month.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 4th, 2023 – Sponsored content –
 (Tim Foster, Unsplash. https://unsplash.com/photos/A-rAZGIE2pA) 4 Sept. 2023. Cannabis dispensaries have plenty to say about the supposed benefits of CBD, THC, and other cannabinoids. Great marketing can go a long way toward luring customers in to try something new. You can also often decide to buy CBD and THC goods based on anecdotal evidence.
There is nothing wrong with buying cannabis goods without reading a research paper or talking to a scientist. However, you might be more inclined to use CBD when you learn about some of these great scientific discoveries below.
It May Alleviate Pain
When you start researching the differences between CBD vs. THC gummies and which CBD products are the most highly recommended, you might encounter research about its pain relief potential. After all, many people buy goods for this very reason.
It’s great to discover that researchers are now studying CBD’s potential for pain relief. In just a few years, they have made some promising discoveries. A 2020 study exploring CBD use for chronic pain found that CBD treatment ‘significantly reduced pain’ on a visual analog scale. A New Zealand study was also mentioned in this research piece. It stated that CBD treatment in 400 chronic pain patients improved pain and quality of life. Multiple in vitro and animal studies have also supported this theory.
It’s a Potential Anxiety Disorder Treatment
Thousands of people struggle to manage their anxiety on a daily basis. While conventional anxiety medications are available, some people seek more natural alternatives. Many people state that CBD helps them feel more relaxed and in control. Now, some studies are starting to validate that anecdotal evidence. One 2015 study found that preclinical evidence supports CBD at 300mg-600mg doses as a treatment method for:
- Generalized anxiety disorder
- Panic disorder
- Social anxiety disorder
- Obsessive-compulsive disorder
- Post-traumatic stress disorder
It Could Protect Against Microbes
Scientists have been exploring CBD for cancer, pain, mental health, and other ailments. However, researchers are now looking beyond those popular study areas. In 2019, it was being investigated for potential benefits as an antibiotic. Scientists have wondered whether it’s as effective as antibiotics against microbes like Staphylococcus and Streptococcus.
The research found that CBD was effective against gram-positive bacteria and worked as fast and effectively as two antibiotic types. The authors concluded that CBD’s anti-inflammatory effects, safety data, and various delivery routes make it a ‘promising new antibiotic’ worth investigating.
It Might Alleviate Migraines
Migraines affect millions of people worldwide and can be debilitating. Many people also don’t experience relief using off-the-shelf pharmaceutical products. However, some migraine sufferers are experiencing relief from medical cannabis.
Current studies suggest that medical cannabis might be effective in decreasing pain intensity. It might also reduce people’s reliance on analgesics. In those same studies, some patients experienced physical and mental health improvements.
We can take cannabis dispensaries’ word for it that we’ll enjoy our experience with CBD and THC goods. However, it’s always nice when scientists back up our beliefs. If you’ve used CBD for these reasons above, you might enjoy peace of mind knowing scientists have found the possible benefits you’ve known all along.
* * *
By Alan, on September 1st, 2023 – Contributed content –
 (pixelcreatures, Pixabay) 1 Sept. 2023. Protecting your business in today’s technology-dominated environment has never been more crucial. Gone are the days when simply installing burglar alarms or CCTV cameras was enough – now, companies must not only defend against physical dangers like theft and vandalism but also deal with cyber risks like data breaches, ransomware attacks and phishing attempts for successful operation.
This comprehensive guide offers business owners an all-encompassing approach to protecting their enterprise, covering areas like physical security, digital protection and legal advice.
Installation of an Effective Physical Security Surveillance System
Combine CCTV cameras, motion detectors and access control systems into an efficient surveillance network for real time monitoring with all records safely stored away in one central repository.
Hiring Security Personnel
Hiring trained security staff who can monitor the premises and respond swiftly in case of security threats will give you peace of mind while assuring safe operations at your facilities.
Safe Perimeter
To protect the safety and wellbeing of your business premises, install secure fencing, gates and barriers at its perimeter in order to defend it against external attacks.
Update Your Software Regularly
Maintaining regular software and system updates provides extra defense from security threats that arise over time.
It is a good idea to implement an advanced firewall solution in order to stop unwanted guests from accessing your network unauthorized. You can enhance this security by using up-to-date antivirus software on all computers and networks connected to them in order to safeguard them against cyber threats, such as malware and ransomware.
Training of Employees
Train your staff on how to recognize and respond to cyber attacks such as phishing emails or ransomware attacks.
Data Encryption
Encrypting sensitive information helps safeguard it against unwarranted access and protect its confidentiality.
Legal Protection
Secure yourself against risks like theft, vandalism, fire and cyber attacks which might harm your company with comprehensive business insurance policies.
Contracts and Agreements
Ensure all your agreements and contracts comply with legal standards in order to protect your business interests and remain valid.
Intellectual Property Protection
Protect your intellectual property with trademark, patent and copyright registrations to safeguard it for years to come.
Implement Document Management Software
Document management software provides a safe way of organizing, storing, tracking and retrieving electronic documents in an organized fashion. A sound document management system should help centralize documents in one central place while controlling access and providing an audit trail of who viewed or modified what documents at what times. You may want to look into document management benefits to help your firm.
In addition, good document management systems make retrieving files simpler; foster collaboration among employees while meeting legal obligations regarding document retention/destruction obligations.
Employee Wellness and Mental Health
For maximum employee productivity and happiness, wellness programs designed to address physical, mental and stress management health are a necessity. Encourage an ideal work-life balance with regular breaks and offer mental health support services within an upbeat workplace culture thus creating safe working conditions in which mental illness support services can thrive!
Training Your Employees for Cybersecurity
Conducting cybersecurity training sessions for employees is vital in keeping them abreast of current threats, how best to deal with them, password management best practices and internet usage policies as well as understanding why any suspicious activities must be reported immediately.
Conclusion
Protecting your business requires taking several measures that cover physical security, digital asset protection and legal compliance. By being proactive about any threats that might emerge and taking an integrated approach to address all potential dangers you can create an ideal environment for its growth and success – never underestimate its significance.
Adequate protection requires more than just once; rather it requires ongoing observation and adjustment – be vigilant, stay secure.
Editor’s note: Readers are also urged to make security assessments reflecting their own particular threats, and take the necessary precautions.
* * *
By Alan, on August 28th, 2023  (Shutterbug75, Pixabay. https://pixabay.com/photos/camera-aperture-digital-camera-dslr-1239384/) 28 Aug. 2023. Science & Enterprise is taking off the last week of August to prepare the annual photography exhibit at National Press Club in Washington, D.C. and brief travel. We will resume our regular editorial posts after Labor Day in the U.S. on 5 Sept.
* * *
By Alan, on August 26th, 2023 26 Aug. 2023. The success this week of the Chandrayaan-3 mission from India to land in the moon’ south polar region provides a chance to look ahead to moon missions planned for the foreseeable future. The business research company Statista published an infographic this week with moon missions announced through 2030.
According to Statista, the Jaxa space agency from Japan plans a landing later this year, while NASA from the U.S. also expects to launch an orbital mission to the moon this year. Next year, however, looks busier with Jaxa, NASA, and companies under contract to those agencies planning to orbit or land payloads or rovers on the moon. In addition, China plans a complex landing, rover, and drone mission in 2024.
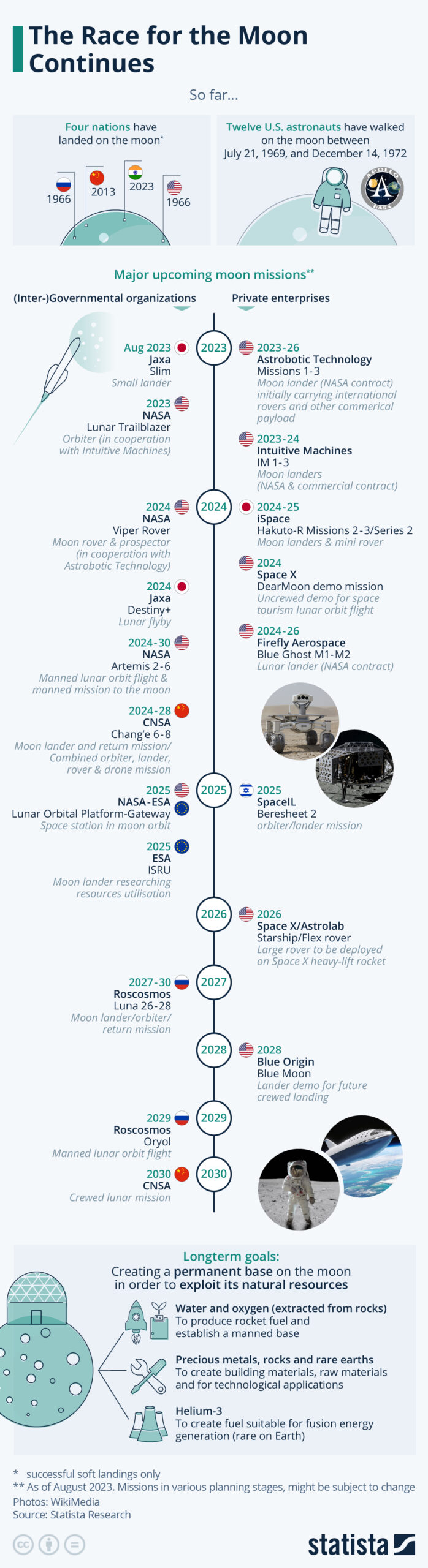 (Statista) More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 25th, 2023  (commonfund.nih.gov) 25 Aug. 2023. A clinical trial is underway testing a new type of therapy its developer says addresses damage to nerve cells associated with a number of neurological disorders. Nura Bio Inc. in South San Francisco says the trial, the company’s first, is evaluating its lead product code-named NB-4746, but the study is not yet registered with ClinicalTrials.gov.
Nura Bio is a five year-old company discovering treatments for neurological conditions associated with degenerating axons, the long fiber tails in nerve cells or neurons that transmit electrical signals to other neurons. The Nura Bio process seeks to prevent and repair damage to axons from Wallerian degeneration, where a key protein needed for healthy neurons called nicotinamide mononucleotide adenylyltransferase 2 or NMNAT2 is missing, causing neurons to lose their signaling ability. Another protein, coded by the Sterile Alpha and TIR Motif Containing 1 or SARM1 gene, is triggered by nerve cell injury, believed to encourage further damage to axons, and also associated with Wallerian degeneration.
The company’s technology is based on research into Wallerian degeneration by its scientific founders Marc Freeman at Oregon Health and Science University and Steven McKnight at University of Texas Southwestern Medical Center. Freeman’s lab investigates damage to axons resulting in malfunctioning neurons and supporting glial cells, while the McKnight lab studies the chemistry of molecules associated with neurodegenerative diseases, focusing on compounds that limit nerve cell degeneration. As reported by Science & Enterprise in July 2020, Nura Bio was formed by Bay area venture investors, which staked the company to $73 million in its first venture round.
Effective in central and peripheral nervous systems
Nura Bio says NB-4746 acts as a SARM1 inhibitor to protect against damage to axons from Wallerian degeneration associated with several neurological conditions: amyotrophic lateral sclerosis or ALS, multiple sclerosis, traumatic brain injury, and nerve damage from chemotherapy. The company says its preclinical studies show NB-4746 can be given as an oral drug and still be effective in brain cells, as well as with neurons elsewhere in the central and peripheral nervous system, as well as the eyes.
The clinical trial, says Nura Bio, is a phase 1 or early-stage study with healthy volunteers, randomized to receive single and multiple ascending doses of NB-4746 or a placebo. Participants will be assessed for safety and tolerability of NB-4746, as well as chemical activity in the body. Numbers of participants in the trial and study sites were not disclosed.
“The initiation of the phase 1 trial of NB-4746 marks Nura Bio’s transition to a clinical-stage company,” says the company’s chief scientist Shilpa Sambashivan in a Nura Bio statement. She also notes the company is working on other other similar therapies. “We also continue advancing additional molecules through our pre-clinical pipeline,” adds Sambashivan, “with the goal of delivering novel and potentially life-changing neuroprotective medicines.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 24th, 2023  Salmonella Typhi bacteria responsible for typhoid fever (Public Health Library, CDC.gov) 24 Aug. 2023. A health research foundation grant is funding development of a vaccine protecting against typhoid fever, given in a patch from from a spring-loaded device. Vaxxas Inc. in Cambridge, Massachusetts and Hamilton, Australia is receiving a $3.7 million award from the Wellcome organization, a charitable health research and advocacy group in the U.K., and developing the typhoid vaccine patch with SK bioscience in Seongnam-si, South Korea.
Vaxxas develops a device that administers vaccines from a small, single-use patch made with microscale needles, about 0.25 mm in length, coated with a dry form of the vaccine. The needles, says Vaxxas, penetrate the very outer skin layers, enough to stimulate an immune response. The high-density micro-array patch or HD-MAP, is placed on the skin from a spring-loaded dispenser that also seals the patch before use. The dried vaccine applied to the microscale needles on the patch do not need refrigeration, enabling storage and transport in ambient temperatures, an advantage over conventional syringe-administered vaccines.
As reported in Science & Enterprise, Vaxxas is testing the HD-MAP device in clinical trials with vaccines for influenza and Covid-19, with results presented in June showing the Covid-19 vaccine patch is well-tolerated and induces an immune response. Results of a separate trial reported earlier this year shows the HD-MAP can be administered by patients themselves with results similar to administration by clinicians.
Parts of a pathogen to generate an immune response
In their collaboration, Vaxxas is testing a conjugate vaccine made by SK bioscience for typhoid fever formulated for HD-MAP administration. Typhoid fever, says World Health Organization, is an infection from Salmonella Typhi bacteria, spread through contaminated water and food, and often found in locations without adequate sanitation or safe drinking water. WHO says some 9 million people are affected by typhoid fever each year with symptoms including fever, nausea, abdominal pain, headache, and diarrhea that can become life-threatening.
WHO recommends conjugate vaccines to protect against typhoid fever infection since they can be given to children as young a six months and adults in some cases up to 65 years of age. Conjugate vaccines use one or more parts of a pathogen to generate a strong immune response. SK bioscience developed its SKYTphoid conjugate vaccine with International Vaccine Institute and is already approved for export, according to the company. In the collaboration with Vaxxas, the two companies are reformulating the SKYTphoid vaccine for use with the HD-MAP device.
The Wellcome organization is providing $3.67 million for the two-year initiative, covering preclinical studies of a typhoid fever vaccine delivered with an HD-MAP device, leading to an investigational new drug application, in effect a request to regulatory authorities to begin clinical trials. The funding also covers an early-stage clinical trial.
“To help protect more people at risk from deadly diseases like typhoid fever,” says Wellcome organization senior research manager Pierre Balard in a Vaxxas statement released through BusinessWire, “new vaccine innovations are needed to improve access and ensure equitable coverage.” Ballard adds, “With the potential to overcome some of the most enduring barriers to vaccine access in lower income countries, this product could be a vital addition to our global toolkit.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 23rd, 2023  (Gerd Altmann, Pixabay) 23 Aug. 2023. The Department of Health and Human Services is allocating $100 million for venture investments in new technologies to prepare for future threats from Covid-19. The funds are part of a $1.4 billion package for the NextGen project, announced yesterday by HHS’s Administration for Strategic Preparedness and Response for a series of clinical trials, a new monoclonal antibody for prevention, and a challenge competition.
HHS is directing the $100 million of the NextGen package to the Global Health Investment Corporation, a not-for-profit organization that applies a venture investing model to fund what it calls high-impact biomedical innovations. GHIC works through partnerships with government and private entities including the Biomedical Advanced Research and Development Authority or BARDA, an agency of HHS responsible for counteracting public health threats including emerging infectious diseases. In 2021, BARDA and GHIC established a 10-year program to create a venture portfolio for global health security, to advance new technologies in vaccines, diagnostics, therapeutics, and manufacturing processes.
While vaccines and therapies for Covid-19 infections receive most of the public attention, the BARDA-GHIC partnership also supports the underlying infrastructure needed for delivering these products to clinics and patients. One of the program’s portfolio companies is National Resilience Inc., known simply as Resilience, a company providing biotechnology manufacturing and distribution services. Science & Enterprise reported on the company’s initial operations in Nov. 2020 and a few times since.
Crowd-sourced competitions for new ideas
Another part of the NextGen project involving entrepreneurs is a challenge competition for new technologies applicable to Covid-19 preparedness. BARDA and Johnson & Johnson Innovation, a division of the health care products company, sponsor the Blue Knight program that conducts crowd-sourced competitions for new ideas in combating threats to public health, including infectious diseases. The NextGen initiative is allocating $10 million to encourage fresh ideas from entrepreneurs, university labs, and student teams.
In May 2023, BARDA and J&J Innovation held a $10 million Blue Knight challenge competition for vaccines, therapies and related technologies addressing potential health security threats, with prizes of up to $1 million for preclinical solutions and $3 million for entries that reach clinical development. Ten companies received awards from the competition.
The majority of new NextGen funds, $1 billion of the $1.4 billion total, is devoted to new mid-stage clinical trials testing vaccine candidates against emerging Covid-19 variants. The trials are conducted by companies taking part in BARDA’s clinical studies network established to streamline trial start-up and execution for product candidates addressing security threats from nuclear, chemical, and biological weapons as well as infectious diseases.
NextGen is directing another $326 million to Regeneron Pharmaceuticals Inc. in Tarrytown, New York for a new type of synthetic monoclonal antibody to prevent infections from SARS-CoV-2 viruses responsible for Covid-19 disease. The contract with BARDA calls for Regeneron to initially design and propose a monoclonal antibody candidate for review by BARDA and the company for subsequent development, testing, and regulatory review. Regeneron expects the new antibody candidate to begin clinical trials next year.
More from Science & Enterprise:
Disclosure: The author owns shares in Johnson & Johnson.
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 22nd, 2023  Female Anopheles albimanus mosquito, responsible for spreading malaria (James Gathany, Pixnio.com) 22 Aug. 2023. A company creating a digital process to monitor farm insect pests is receiving funds to expand its technology for tracking and counting mosquitoes as a general public health threat. National Science Foundation is awarding FarmSense Inc. in Riverside, California $275,000 for a working system that accurately and inexpensively counts and classifies mosquitoes in a specified area.
FarmSense is commercializing research by the company’s scientific founder Eamonn Keogh, a computer science professor at University of California in Riverside, who studies analytics of time-series statistical data. Among the practical applications of Keogh’s research, is analysis of sensor data for identifying and quantifying insects in a region. Most current methods for quantifying insect populations, says the company, use mechanical traps similar to flypaper that require manual collection and counting.
FarmSense is a seven year-old company that offers electronic insect monitoring systems for farms that collect data from electronic monitoring stations that lure insects in a growers fields. Those stations, called FlightSensors, use LED lights and optical sensors to identify flying insects from their wing beating patterns, with the insect data and location coordinates of the station sent via Wi-Fi or cell networks and a server to the cloud for analysis with machine-learning algorithms. Growers using the FarmSense service receive real-time reports of insect pest activity in their fields.
The NSF grant supports further development of the FarmSense technology to identify and track numbers of mosquitoes in a specified area for public health surveillance. Mosquitoes are known to spread viral and parasitic diseases such as West Nile, Zika, and malaria, but measures to control mosquitoes by public health authorities first need accurate data on numbers and precise species at various locations in their districts. Better identification and counts can also help assess control measures against mosquitoes.
Stations activated by the presence of insects
In addition, the award calls for more autonomous mosquito trap stations that work like FlightSensors to lure insects into the stations for identification and counting. NSF calls for upgraded data-collection stations activated by the presence of insects rather than the current method of continuous operation. The new stations are expected to reduce use of carbon dioxide for luring mosquitoes to the stations, extend the batteries powering the stations, and reduce staffing needed for maintenance.
“I’m confident,” says FarmSense co-founder and CEO Leslie Hickle in a company statement, “that the capabilities of our technology and FlightSensor device will provide the necessary data to effectively serve both the commercial agriculture industry as well as provide important public health information. This is one of those rare opportunities where technology benefits both agriculture as well as public health, generally separate markets, to enhance precise measures for insect suppression.”
NSF is making the award under its Small Business Innovation Research or SBIR program, which the agency says sets aside more than $200 million a year for some 400 U.S. start-ups. SBIR grants are usually made in two phases, with the first part to demonstrate technical and commercial feasibility, and the second part to advance and scale-up the technology for commercial markets. The new FarmSense grant is a phase 1 award.
FarmSense is no stranger to the SBIR program at NSF. In 2021, the agency awarded the company an $858,541 SBIR award for developing more accurate and autonomous insect trap and counting stations, as well as supporting algorithms.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|










 RSS - Posts
RSS - Posts
You must be logged in to post a comment.