15 June 2021. Two biotechnology companies are designing therapies to deliver edited genes to treat Friedreich’s ataxia and amyotrophic lateral sclerosis, or ALS. Financial terms of the agreement between Crispr Therapeutics in Zug, Switzerland and Cambridge, Mass., and Capsida Biotherapeutics Inc. in Newbury Park, California were not disclosed.
The two companies are collaborating on treatments that work inside the body, called in vivo therapies, for these neurological diseases that deliver functioning edited genes with engineered adeno-associated viruses. Friedreich’s ataxia is a rare disorder caused by a mutation in the FXN gene that codes for the protein frataxin. People inheriting two defective copies of the FXN gene, one from each parent, are likely to develop the disease, which allows nerve fibers in the spinal cord and peripheral nerves to degenerate and become thinner.
ALS, also known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder where neurons or nerve cells controlling muscles in the body begin to waste away, and can no longer send or receive signals from the brain or spinal cord. As the nerve cells stop functioning, the muscles in the limbs, and later speech and breathing muscles, begin weakening and eventually stop functioning. Most people with the disease die of respiratory failure.
Improved AAV targeting limits off-target effects
Crispr — short for clustered, regularly interspaced short palindromic repeats — makes it possible to edit genomes of organisms harnessing bacterial defense mechanisms that use RNA to identify and monitor precise locations in DNA. At Crispr Therapeutics and in most other cases, the actual editing is done by Crispr-associated protein 9, or Cas9, enzyme that programs RNA to cut DNA at precise points in genomes, making it possible to delete, insert, or correct defects in human genomes.
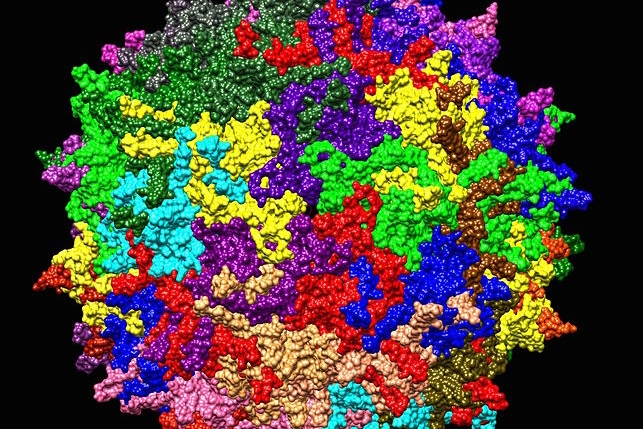
Capsida Biotherapeutics creates engineered adeno-associated viruses to deliver gene therapies. Adeno-associated viruses or AAVs are benign, naturally occurring microbes that infect cells, but do not integrate with the cell’s genome or cause disease, and generate at most a mild immune response. Capsida says it modifies AAVs to reduce adverse events from gene therapies with better targeting that limits off-target effects and also makes possible lower treatment doses.
Crispr Therapeutics and Capsida are partnering on design, development, and production of in vivo therapies for the two neurological diseases. Crispr Therapeutics is taking the lead on a Friedreich’s ataxia treatment, while Capsida is leading on a therapy for familial ALS, an inherited form of the disease affecting five to ten percent of all cases. Each company has the option of co-developing or co-commercializing the other partner’s treatment candidates, where the partners would share R&D and commercialization costs, as well as profits on the joint products. In addition, Capsida is responsible for process development and manufacturing of clinical-stage candidate products, and has an option for manufacturing commercial products once they reach that stage.
“The combination of Capsida’s AAV engineering platform and Crispr Therapeutics’ gene-editing platform has the potential to enable transformative gene-edited therapies for patients with neurological diseases,” says Crispr Therapeutics CEO Samarth Kulkarni in a statement.
More from Science & Enterprise:
- Crispr Treatment Leads to Durable Cholesterol Reduction
- Gene Therapy Company Starts-Up, Gains $200M
- Biotechs Partner on Gene-Edited Cancer Cell Therapies
- AI Gene Therapy Platform Gains $100M in Early Funds
- Start-Up Designing Synthetic DNA for Gene Therapies
* * *

 RSS - Posts
RSS - Posts
[…] Biotechs Partner on Crispr Therapies for Neuro Disorders […]