Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on November 17th, 2023  Islet cells from the pancreas (Masur, Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Pancreaticislet.jpg) 17 Nov. 2023. Health authorities in Sweden authorized the start of a clinical trial testing insulin-producing cells from donors engineered to prevent immune reactions in people with type 1 diabetes. Sana Biotechnology Inc. in Seattle says the Swedish Medical Products Agency authorized the trial, a first-in-human study of donated and immune-safe islet cells, held at Uppsala University Hospital.
The trial plans to test an experimental treatment for type 1 diabetes code-named UP421. Type 1 diabetes is an autoimmune variation of the disease where healthy islet cells in the pancreas are attacked as if they were foreign pathogens preventing production of insulin that processes glucose or sugar in the blood. People with type 1 diabetes need to continually monitor their glucose and take insulin regularly to keep blood glucose at safe levels. Centers for Disease Control and Prevention says some 5.7 percent of adults in the U.S. diagnosed with diabetes have type 1.
UP421 aims to provide functioning islet cells from donors to people with type 1 diabetes, without asking recipients to take drugs that suppress the immune system to prevent dangerous immune reactions. Sana Bio says in this trial islet cells from recently deceased individuals, called allogeneic cells, will be engineered with the company’s hypoimmune technology. That technology, says Sana Bio, disrupts expression of human leukocyte antigens, key proteins in the immune system that distinguish between a cells and tissue native to a person and those from outside. The company says hypoimmune also over-produces proteins called CD47, found on many cell surfaces, that stop macrophage immune cells from attacking.
Remove immunosuppression from allogeneic cell transplant
Uppsala University cell biologist Per-Ola Carlsson who studies clinical solutions to type 1 diabetes is leading the trial. “We have performed approximately 165 allogeneic, primary islet cell transplants,” says Carlsson in a Sana Biotechnology statement, “and have seen the benefits for patients, but the complications of immunosuppression inhibit broader use of this procedure. Combining Sana’s hypoimmune technology with primary islet cell transplantation therapy can generate important first-in-human data that may be a step to removing immunosuppression from allogeneic cell transplant in the type 1 diabetes setting.” The research team hopes to report proof-of-concept results for UP421 later this year or in 2024.
Sana Bio says its preclinical research indicates hypoimmune technology has the potential for donated cells to evade immune-system recognition, enabling islet cells in this case to graft in the pancreas, survive, and begin producing C-peptides that enable production of insulin. “Sana’s hypoimmune platform,” notes company CEO Steve Harr, “has shown the potential to evade both allogeneic and autoimmune rejection in preclinical models, and we look forward to seeing if these insights translate into patients, providing a path to cell transplantation without immunosuppression.”
The company expects findings from the trial will help guide development of SC451, Sana Biotechnology’s engineered cells for type 1 diabetes. SC451 applies the hypoimmune process to islet cells derived from induced pluripotent or adult stem cells rather than donated cells. The five year-old company is developing engineered donated and stem cell treatments for cancer, autoimmune disorders, and neurological diseases. Sana Bio is spun-off from labs at Harvard University. Science & Enterprise reported on the company’s start-up in Mar. 2019 and initial public offering in Feb. 2021.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 16th, 2023  (Gerd Altmann, Pixabay. https://pixabay.com/illustrations/digitization-particles-smartphone-7261158/) 16 Nov. 2023. A biotechnology company demonstrated its technology for designing entirely new types of therapeutic proteins based on a generative artificial intelligence model. Generate:Biomedicines in Somerville, Massachusetts describes its Chroma generative A.I. model in yesterday’s issue of the journal Nature, and is making the model available as open source through GitHub.
Generate:Bio is a five year-old company that designs and develops therapeutic proteins from scratch to meet specific chemical and medicinal properties. The company says its Chroma platform is based on an A.I. neural network or machine learning model trained on amino acid sequences, three-dimensional structures, and statistical patterns of proteins in nature. The model next applies diffusion principles for generating new protein sequences and structures, starting with simple data that then adds more complexity with each following step to achieve the desired functions. In addition, says Generate:Bio, the Chroma technology is programmable, in that the model can be steered to design novel protein structures and sequences that meet chemical and therapeutic objectives specified by the user.
The company says its process can design protein molecules from short peptides to more intricate enzymes, antibodies, and gene therapies. Generate:Bio is developing several of its own protein therapeutics for immunology, cancer, and infectious diseases, with a drug addressing SARS-CoV-2, the virus responsible for Covid-19 infections, in an early-stage clinical trial. The company is also collaborating with M.D. Anderson and Roswell Park cancer centers on therapeutic protein discovery and drug maker Amgen on a number of undisclosed disease targets.
Programming protein output with natural language prompts
In the Nature paper, a team led Generate:Bio co-founder and chief technology officer Gevorg Grigoryan analyzed 120,000 novel protein designs generated by the Chroma model, both single-chain and more complex proteins, but not programmed for specific therapeutic functions. The analysis shows the novel protein designs match many of the properties of natural proteins in the Protein Data Bank, a training source of the company’s algorithms. The researchers also demonstrated programmable structure, folding, sizing, and shaping features of Chroma, even creating proteins shaped like Roman alphabet and Arabic number characters. In addition, the team displayed the ability of programming Chroma output with natural language prompts.
The researchers validated the Chroma model by generating 310 novel designs and testing the proteins they produced on features such as solubility, melting temperature, and expression in E. coli bacteria, a commonly used model species in biology. And the team also produced proteins meeting specified chain lengths, from 100 to 950 amino acids. The authors say their tests show Chroma can produce very large and complex protein designs with standard A.I. graphic processing chips in a few minutes.
Grigoryan — also a professor of computer science and biology at Dartmouth College — says in a Generate:Biomedicines statement, “we have demonstrated that fundamentally new molecular concepts can now be generated at will and used to create novel proteins with a diverse set of desired properties.” Plus, the company is making the Chroma model code available as open source on the GitHub web site. “By making the code open source,” adds Grigoryan, “we’re proud to share this breakthrough in generative biology with the world to help advance our shared goals of fundamentally changing the way protein therapies are created.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 15th, 2023  Cannabis plant (Michael Fischer, Pexels.com) 15 Nov. 2023. A clinical trial in a small group of participants suggests a cannabinoid drug is safe and well-tolerated, and reduces agitation among patients with Alzheimer’s disease. Initial results of the trial are published today in a news release from the biotechnology company SciSparc Ltd., developer of the experimental drug tested in the trial, and are not yet peer-reviewed.
SciSparc, in Tel Aviv, Israel, develops treatments for central nervous system diseases with cannabinoids, derived from the cannabis sativa plant. Cannabinoids are substances that contain varying amounts of tetrahydrocannabinol or THC, the mind-altering compound in cannabis, as well as cannabidiol and some 100 other chemicals. Research with cannabinoids indicates some of these compounds have beneficial effects with few adverse effects for treating mental health disorders, with the Food and Drug Administration approving cannabinoid drugs for some forms of epilepsy, and nausea and vomiting in cancer patients undergoing chemotherapy.
SCI-110 is SciSparc’s lead product, in clinical trials for tourette syndrome, obstructive sleep apnea, and now agitation from Alzheimer’s disease. Some people with Alzheimer’s disease and other dementias experience behavior changes including increased fidgeting, restlessness, and agitation. The symptoms may result from changes in the environment of patients such as new caregivers, or fear and fatigue from the disease itself. But the agitation can be damaging to the well-being of both the patient and caregivers, with few options other than calming the patient, removing possible triggers from the environment, and a few medications.
Older adults with Alzheimer’s disease experiencing agitation
SCI-110 is a combination of the cannabinoid drug dronabinol made with synthetic THC, approved by FDA to treat nausea and vomiting in chemotherapy recipients, and the endocannabinoid compound palmitoylethanolamide, or PEA. Endocannabinoids are lipid or natural oil compounds that maintain and protect the central nervous system, by producing enzymes for synthesizing and degrading endocannabinoids as well as by engaging cannabinoid receptors. SciSparc produces SCI-110 as an oral drug.
The clinical trial is a mid-stage study testing SCI-110 among up to 20 patients in an Alzheimer’s care center in Tel Aviv. Taking part in the trial are older adults, age 60 to 85, experiencing agitation but not responding to their current medications. Participants are given SCI-110 twice a day for 64 days with the dose of dronabinol in the drug increased every three days up to 12.5 milligrams. There is no control or comparison group.
The study team is looking mainly for safety indicators in reports of adverse effects and the number of dropouts from the study. But the researchers are also tracking behavioral indicators including a standard agitation assessment inventory, a sleep disorders index, a feeding and eating evaluation scale for dementia patients, measures of impairment, and use of rescue medications.
SciSparc says results from 18 participants show the trial achieved its primary objectives of safety and tolerability, but does not give quantitative data on adverse effects or study drop outs. The company did report a 23 percent decline in agitation symptoms among participants, according to scores on the standard Cohen Mansfield Agitation Inventory. And the scores on the Edinburgh Feeding Evaluation in Dementia Scale, says SciSparc, show fewer eating difficulties. No other behavioral results are reported.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 14th, 2023  Pseudomonas Aeruginosa bacteria (Public Health Image Library, CDC) 14 Nov. 2023. A company formed earlier this year is developing precision treatments for bacteria resistant to conventional antibiotics, and raising £4.3 million ($US 5.4 million) in seed funds. Glox Therapeutics in Glasgow, Scotland, U.K. is spun-off from labs at University of Glasgow and University of Oxford studying engineered proteins to attack resistant microbes.
Glox Therapeutics designs new treatments for infections from gram-negative bacteria that mutate and are difficult to eradicate with today’s antibiotics. Antibiotic resistance is a continuing threat to public health worldwide, which according to the U.S. Centers for Disease Control and Prevention was responsible for 1.2 million deaths in 2019, citing data from The Lancet. Gram-negative bacteria, those with a protective outer membrane, are particularly difficult to control and more likely to become resistant. “Gram” refers to a classification where bacteria either keep (gram-positive) or shed (gram-negative) a test stain on their outer cell membranes.
The technology offered by Glox Therapeutics is based on research by its scientific founders Colin Kleanthous, professor of biochemistry at Oxford and microbiology professor Daniel Walker at University of Glasgow, now at University of Strathclyde, also in Glasgow. Kleinthous and Walker study protein interactions with bacteria, particularly protein molecules called bacteriocins, produced naturally by bacteria to ward off competing microbes. The company is developing synthetic bacteriocins targeting gram-negative bacterial species currently resistant to most antibiotics, but designed not to harm the human microbiome, communities of helpful microbes in the body.
Treatment for ventilator-associated pneumonia
Glox Therapeutics says its first products are addressing Pseudomonas aeruginosa or P. aeruginosa and Klebsiella pneumoniae bacteria that are on the World Health Organization’s list of critical drug-resistant bacteria. The company’s expects its lead product to be an intravenous formulation of bacteriocins to treat pneumonia that develops in the lungs of people on ventilators, where bacteria enter through the ventilator tube. And among the company’s bacterial targets are Escherichia coli or E. coli and Acinetobacter baumannii bacteria, also on the WHO critical drug-resistant bacteria list, with treatments for other types of pneumonia, sepsis, and lung infections in people with cystic fibrosis and chronic obstructive pulmonary disease or COPD.
“Our mission,” says Glox Therapeutics CEO James Clark in a company statement, “is to provide physicians and patients with highly potent, targeted antimicrobial therapies that can kill antibiotic-resistant bacteria for which there are diminishing options available for treatment.”
Glox Therapeutics is raising £4.3 million in seed funds, led by the venture investment arm of drug maker Boehringer Ingelheim and Scottish Enterprise that supports technology start-ups in Scotland. The biotechnology company plans to use the proceeds to establish labs in Glasgow and Oxford, and add key staff. “This will enable us to establish laboratories and attract top-tier talent,” adds Clark, “and I’m delighted to lead the team as we embark on our pioneering bacteriocin development program, with the first target being P. aeruginosa.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 13th, 2023  Adeno-associated virus diagram (Lawrence Berkeley National Lab) 13 Nov. 2023. A developer of synthetic antibodies for neurodegenerative disorders delivered like gene therapies is raising €129 million ($US 138 million) in its first venture funding round. VectorY Therapeutics BV in Amsterdam, The Netherlands plans to initially use the proceeds to advance its lead program, a treatment to delay progression of amyotrophic lateral sclerosis or ALS.
VectorY Therapeutics is a three year-old biotechnology enterprise founded by the Dutch life science venture investor Forbion to advance gene therapies for degenerative nervous system diseases such as ALS. The company designs and delivers synthetic antibodies aimed at clearing specific toxic proteins or lipids in brain cells or elsewhere in the central nervous system. VectorY says its antibodies are fused with degrons, amino acid tags identifying unique protein degradation sequences that provide precise targeting.
The company delivers these payloads with adeno-associated viruses or AAVs, benign viruses with single strands of DNA that allow for adding genetic materials. AAVs have become delivery workhorses for gene therapies. However, VectorY Therapeutics alters the capsid or outer shell of its AAVs to help penetrate the blood-brain barrier, a normally protective mechanism in the brain that also limits the reach of many medications. In addition, the company says it built a manufacturing platform based on baculoviruses that in nature infect insects, but are engineered in this case to reliably produce large-scale quantities of AAV vectors.
Targeting toxic proteins in brain cells
The lead product at VectorY Therapeutics, code-named VTx-002, applies the company process to address ALS, also known as Lou Gehrig’s disease that occurs when motor neurons in the central nervous system begin wasting away. People with ALS lose their ability to first control voluntary movements, such as standing and walking, then often progresses to walking, chewing, and swallowing, and eventually breathing. VTx-002 targets misfolded TAR DNA-binding protein 43 or TDP-43 in neurons associated with ALS, to clear accumulations of toxic misfolded TDP-43 and allow normal TDP-43 to form in its place. Company representatives presented preclinical lab evidence of this process at a scientific meeting in Dec. 2022.
VectorY Therapeutics is raising €129 million in its first venture financing round, called series A, led by life science investors Forbion in Narden, The Netherlands that founded the company and EQT Life Sciences in Amsterdam. Joining the round are MRL Ventures Fund, the corporate venture arm of drug maker Merck, ALS Investment Fund, BioGeneration Ventures, and others. According to Crunchbase, VectorY raised €31 million ($US 38 million) in seed funds in June 2021.
The company plans to first apply the proceeds for advancing VTx-002. “The investment,” says VectorY Therapeutics CEO Sander van Deventer in a company statement, “will enable us to advance our lead program VTx-002, a potentially disease-modifying therapy for ALS, into clinical development. Our program is uniquely positioned to address TDP-43 pathology, which underlies the disease in the vast majority of ALS patients. The series A will also support advancement of additional pipeline programs targeting proteinopathies in neurodegenerative diseases demonstrating the broad potential of our platform.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 11th, 2023 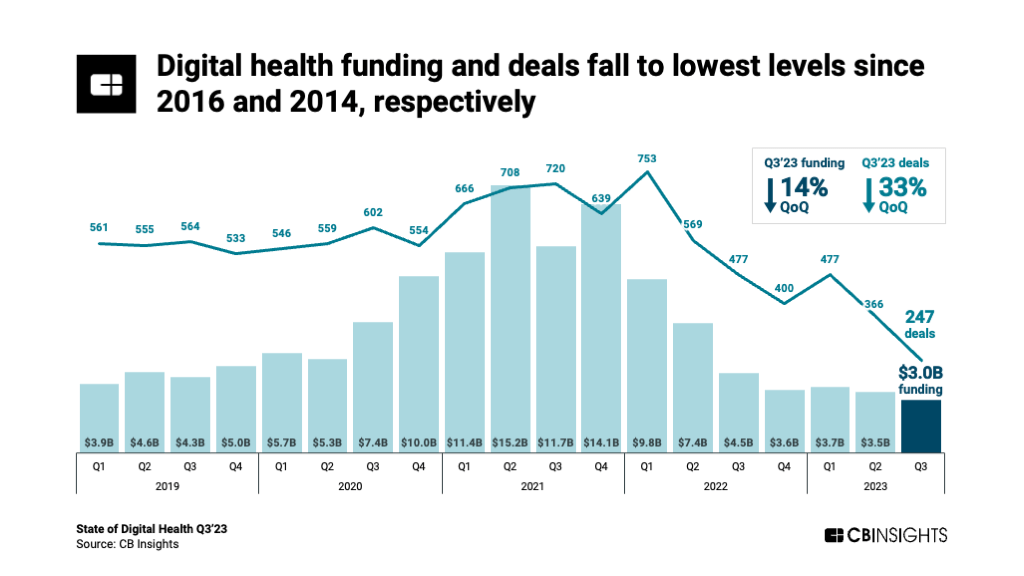 Click on image for full-size view (CB Insights) 11 Nov. 2023. Venture funding for digital health start-ups continued heading down in the third quarter of 2023, reaching low levels not seen in almost a decade. The technology intelligence company CB Insights describes the data for this sector in a report issued in late October (registration required).
CB Insights says companies worldwide developing digital health solutions raised $3 billion in 247 deals from July through Sept. 2023, declines of 14 percent in investment dollars and 33 percent in deal numbers from the previous three months. Moreover, says CB Insights, the $3 billion in investments is the lowest quarterly total since 2016 and the 247 transactions is the fewest number of deals in a quarter since 2014.
Start-ups providing digital care delivery and navigation raised $1.3 billion, nearly half of the total dollars invested by this sector, in 105 deals in Q3 2023. Developers of monitoring, imaging, and diagnostics technologies raised $700 million, while drug R&D start-ups attracted $500 million in the third quarter. The continued downward trend for digital health start-ups runs counter to venture funding overall and in life science start-ups that show gains in Q3 from the previous quarter; see the links below.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 10th, 2023  (Martin Slavoljubovski, Pixabay. https://pixabay.com/photos/teeth-brushing-teeth-dentist-4818757/) 10 Nov. 2023. Results of a clinical trial show a peanut allergy treatment formulated as a toothpaste can safely deliver immunotherapy to raise recipients’ peanut tolerance. A representative of Intrommune Therapeutics Inc. in New York, developer of the experimental peanut allergy therapy, is scheduled to present the findings tomorrow at a meeting of the American College of Allergy, Asthma and Immunology in Anaheim, California.
Peanut allergies are among the most common food allergies, where the immune system reacts to proteins in peanuts as a pathogen, with symptoms ranging from runny nose and skin irritation to life-threatening anaphylaxis. The prevalence of peanut allergy is increasing in Europe and North America, reaching 1.4 to 2.0 percent of the population. In Feb. 2020, as reported by Science & Enterprise, the Food and Drug Administration approved the first peanut allergy treatment for children that exposes recipients to increasing amounts of peanut allergens for six months to desensitize the immune system. Otherwise, people with peanut allergies need to take special care not to ingest food made with even a small amount of peanuts.
Intrommune Therapeutics takes a different approach to build peanut tolerance called oral mucosal immunotherapy or OMIT that the biotechnology company says uses mucous membranes in the oral cavity to desensitize the immune system. Intrommune’s lead product, code-named INT301, is formulated as a toothpaste to deliver peanut immunotherapy as part of a person’s daily tooth-brushing routine. INT301, says the company, is designed as a fully-functioning toothpaste with immunotherapy that transfers through the mouth lining to gradually increases users’ peanut allergen threshold, to where symptoms are no longer triggered by accidental exposure.
Ability to ingest peanut protein
The early-stage clinical trial enrolled 32 adults with peanut allergies at two sites in New Jersey. Participants were randomly assigned on a 3-to-1 basis to use INT301 in escalating doses or a placebo toothpaste over 48 weeks. The study team from Intrommune looked mainly for tolerance and adverse effects from using INT301 as well as adherence to the daily tooth-brushing regimen, finding the maximum tolerated dose and exploratory immunoglobulin biomarkers indicating an immune response. In a subset of participants, the team also tested the ability of participants to ingest 300 milligrams of peanut protein, equivalent to 1 to 1.5 peanuts, at the beginning and end of the 48-week test period.
The researchers report all participants were able to tolerate use of INT301 up to the highest dose of the treatment. Participants reported no more than mild temporary adverse reactions, mainly itching around the mouth. Nearly all (97%) adhered to the treatments, with no dropouts from the study. And the team says initial biomarker measures indicate an immune response among INT301 recipients. In addition, the company reports all of those in the small group using INT301 could ingest 300 milligrams of peanut protein at the end of the study, compared to half of those using the placebo.
William Berger, an allergist and consultant to Intrommune Therapeutics who led the study and is presenting the findings, says in a company statement, “OMIT appeared to be a safe and convenient option for adults with food allergies.” Michael Nelson, CEO of Intrommune adds that the company is designing a mid-stage clinical trial to test INT301 among children.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 9th, 2023  (Gerd Altmann, Pixabay. https://pixabay.com/illustrations/artificial-intelligence-brain-think-4694502/) 9 Nov. 2023. A new challenge competition with $1 million in prizes seeks novel techniques for conducting biomedical research with data on human physiology and artificial intelligence. The Complement ARIE challenge — Complement ARIE stands for Complement Animal Research In Experimentation — is sponsored by the NIH Common Fund and conducted for National Institutes of Health by the crowdsourcing company HeroX. Initial submissions are due by 11 Jan. 2024.
With this competition, NIH aims to generate fresh ideas for conducting research on health that takes advantage of rapidly advancing computational technologies such as artificial intelligence and proliferation of detailed data on human physiology. NIH’s Common Fund, the vehicle for financing initiatives that cut across the agency’s institute boundaries, calls these novel ideas New Approach Methodologies or NAMs. The Common Fund hopes the competition can reveal tools for complementing or replacing conventional research models, such as tests with lab animals, to provide precise answers to complex questions about human biology.
The Complement ARIE challenge asks individuals and teams to propose human-based NAMs that can transform the conduct of basic and clinical research. Participants are expected to provide NAMs as detailed solutions that cut across conventional scientific and engineering disciplines to harness computational techniques and microphysiological data to better understand the complexities of human health. In addition, NAMs proposed by participants should be validated with the goal of acceptance by regulatory bodies. The Common Fund expects to use NAMs generated by the competition for its strategic planning, and to model human health and disease across diverse populations.
Up to 20 top solutions receive prizes
The challenge has an accelerated submission and judging schedule that first asks participants to submit a 15-page white paper by 11 Jan. 2024. Proposals should outline new ideas for NAMs covering in-vitro or lab culture studies, as well as computer simulations called in-silico techniques, and studies of molecular interactions in cell-free environments known as in-chemico methods. In addition, participants need to demonstrate the integration of these NAMs, particularly using A.I. for outcomes such as predictive models, real-time data analysis, and population-level or individual patient digital twins.
The challenge guidelines provide details for the format and content of white papers, participation requirements, and judging criteria, plus full eligibility rules. After submission of white papers on 11 Jan. 2024, judges will review proposals through mid-February. Up to 20 top solutions, each receiving $50,000, are expected to be announced later in February.
“NAMs research is at a critical point,” says Douglas Sheeley, acting director of the Office of Strategic Coordination that oversees the NIH Common Fund, in a HeroX statement released through Cision, “where investment and collaboration between multiple sectors could help to enable widespread development and expansion in use of these new technologies.” Sheeley adds, “NAMs fit well as an area for Common Fund investment because they have the possibility to create advancements across broad swaths of biomedical research.”
HeroX is providing the crowdsourcing platform for the Complement ARIE competition. The 10 year-old company provides the mechanisms for conducting idea-generating exercises, such as challenge competitions, for companies and organizations needing crowdsourced solutions for identified problems or issues. In Sept. 2023, Science & Enterprise reported on HeroX providing part of the process for an NIH-sponsored challenge seeking early indicators of Alzheimer’s disease and related dementias using analytics powered by artificial intelligence.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 8th, 2023  Raven (arbyreed, Flickr. https://flic.kr/p/yHVbGg) 8 Nov. 2023. A new-business accelerator program, begun earlier this year to advance and commercialize biomedical research discoveries from academic labs, is receiving a $100 million founding grant. Blackbird Labs in Baltimore, Maryland is collaborating with life science researchers at local institutions including Johns Hopkins University and Lieber Institute for Brain Development, located on the Johns Hopkins campus, as well as University of Maryland in Baltimore.
Blackbird Labs says it aims to close the gap between research breakthroughs in university labs and the marketplace. The organization says it’s identifying new discoveries with market potential, then connecting researchers with life science industry experts to guide their solutions through further development and business creation. Blackbird plans to reduce risks of further development of these discoveries, encourage public-private partnerships, form start-up companies, and provide early capital.
The group says it will initially support research advances in cancer, neurological and genetic diseases, and inflammation and immune disorders. Blackbird, which formed in Jan. 2023, has already started collaborations on an oral drug for Crohn’s disease and colitis, cell-specific gene therapies, a multi-modal treatment for schizophrenia, and a digital technology to ease clinical trial participation by patients.
“Groundwork for the biotech companies of the future”
“We look forward,” says Blackbird Labs CEO Matt Tremblay in an organization statement released through Cision, “to utilizing our collective expertise in academic translation, company creation, and drug development, and leveraging our academic partnerships to lay the groundwork for the biotech companies of the future.” Tremblay was previously chief operating officer at Scripps Research Institute and Calibr, the institute’s drug discovery division.
The Stephen and Renee Bisciotti Foundation is providing a $100 million start-up grant for Blackbird Labs. Steve Bisciotti is owner of the Baltimore Ravens, the city’s NFL franchise, and the foundation’s address is M&T Stadium where the team plays. In March 2023, the foundation’s translational fund awarded seed grants to researchers at Johns Hopkins University for development of a messenger RNA or mRNA gene-therapy booster to address protein deficiencies, chimeric antigen receptor or CAR T-cell therapies for autoimmune diseases, and a vaccine that invokes T-cell responses in the immune system to protect against a range of coronavirus infections.
Christy Wyskiel, executive director of Johns Hopkins Technology Ventures, the university’s technology transfer arm, calls Blackbird Labs, “a unique resource for funding and expertise to help bring Johns Hopkins technologies to market. Its focus on therapeutic assets, one of our strengths, and on Baltimore-based start-ups is well-aligned with our mission.” Wyskiel also advises the university president on innovation and entrepreneurship.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on November 7th, 2023  (NIH.gov) 7 Nov. 2023. A virtual reality program conducted at home is shown in a clinical trial to relieve chronic lower back pain intensity and improve daily living more than a sham V.R. program. Findings from the nationwide clinical trial assessing the therapy designed by the company AppliedVR appear in the 24 Oct. 2023 issue of Mayo Clinic Proceedings: Digital Health.
AppliedVR in Los Angeles applies virtual reality to therapeutics for conditions such as chronic pain, as well as behavioral and mental health disorders. The lead product from the eight year-old company is RelieVRx, a V.R. treatment for chronic lower back pain, performed by the patient at home, once a day for eight weeks. The company says its cognitive behavioral therapy combines diaphragmatic breathing, mindfulness, cognition and emotion regulation with pain education into daily immersive exercises. In addition, the V.R. headset uses a built-in microphone to capture audio of the user’s exhaling patterns as part of biofeedback in the program.
RelieVRx is available by prescription, and cleared by the Food and Drug Administration. The treatments are coded for reimbursement by Centers for Medicare and Medicaid Services and part of the Department of Veterans Affairs’ federal supply schedule.
The authors note that chronic lower back pain is the most prevalent pain condition worldwide, but opioid pain relievers are now too dangerous, resulting in fewer options for patients with the condition. And conventional behavioral therapies require a therapist, which reduces the availability of pain treatments without drugs.
Measures of pain intensity and interference
The clinical trial sampled 1,067 adults, age 18 to 85, with chronic lower back pain in the U.S., recruited by clinics, chronic pain organizations, and online advertisements. The sample was mainly women (72%) with an average age of 51. Participants were all given the same V.R. headsets, but randomly assigned to receive the RelieVRx program or a sham V.R. experience, consisting of non-immersive content such as two-dimensional nature videos and relaxing music. Data were collected directly from participants through online surveys through the Curebase clinical trial platform.
Both the RelieVRx and sham V.R. programs lasted eight weeks, with participants tracked for another 24 months. The study team looked primarily at scores on a standard Brief Pain Inventory questionnaire that measures participants’ evaluations of pain intensity, and pain interference or practical effects of pain on their daily lifestyles, such as mood, walking ability, work, sleep, and relations with others. Researchers also captured other survey data on disability, sleep, anxiety, and depression.
Results show after eight weeks RelieVRx participants record less pain intensity with average scores of two points lower on a 10-point scale than sham V.R. program participants. Likewise, RelieVRx participants report less pain interference after eight weeks than sham V.R. users by an average of 2.3 points on a 10-point scale. In both cases, the differences are large enough for statistical reliability. On the secondary measures, RelieVRx participants say they experience less sleep disturbance, depression, and disability than sham V.R. users. Adverse effects were reported by no more than five percent of RelieVRx or sham V.R. participants, mainly dizziness, vertigo, or nausea.
Beth Darnall, a pain specialist at Stanford University medical school and co-author of the paper who advises AppliedVR, says in a company statement released through Cision that “traditional clinical guidelines have over-relied on medications or surgical procedures, which can be costly and ineffective over the long term.” Darnall adds that the new study, “builds on our previous research at a much larger scale and demonstrates that RelieVRx can be a powerful tool in providers’ tool belts for treating people experiencing chronic low back pain.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.