Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on February 2nd, 2022  (Nattanan Kanchanaprat, Pixabay) 2 Feb. 2022. A not-for-profit organization that sponsors accelerated and high-impact health research worldwide says it gained a new $335 million in funding. Wellcome Leap in Los Angeles says the new funds more than double its original stake of $300 million, when it began operations in 2020.
Wellcome Leap, founded by the British medical research foundation Wellcome Trust, aims to create a model for faster design and development of health breakthroughs, to speed much needed medical discoveries from initial concept through delivery. Wellcome Leap is modeled on the work of the U.S. Defense Advanced Research Projects Agency, or Darpa, that funds ground-breaking discoveries in military-related technologies. But Darpa also brings together key stakeholders and clears away obstacles at the outset to often achieve those breakthroughs in record time.
The group says with that model, its projects bring together basic science with engineering on an accelerated timeline. To speed the start of its work, Wellcome Leap established a worldwide network of institutions committed to using a common master academic research funding agreement, provides all terms and conditions that normally accompany funding documents. Those common provisions include intellectual property, ownership, and publication, agreed upon by 70 academic labs and research centers representing more than 650,000 researchers. Negotiating these terms separately, says Wellcome Leap, can add months to the start of the actual work.
Wellcome Leap has five research initiatives underway. Science & Enterprise reported on its RNA readiness and response, or R3, project that began in July 2021, R3 is a $60 million challenge competition seeking to build a distributed network of facilities to design, develop, and manufacture RNA-based biologic products. The competition, co-sponsored by Coalition for Epidemic Preparedness Innovations or CEPI, uses the semiconductor industry as a model, to encourage more standardized design and production processes, and reduce the reliance on proprietary methods.
Cognitive development in a child’s first 1,000 days
The Multi-Channel Psych project, begun in June 2021, is a $50 million challenge competition, also reported by Science & Enterprise. The initiative seeks reliable biological methods for detecting depression and decision models that connect diagnostics to effective disease treatments. According to Wellcome Leap, current methods for diagnosing and treating depression are at best haphazard with limited results. The challenge seeks more detailed and evidence-based models to connect depression treatments to the patient’s biology. Multi-Channel Psych likewise calls for end-to-end decision models connecting depression diagnostics to treatments.
Other research initiatives underway seek a technology platform to quantitatively profile the state of human tissue and predict changes in that tissue condition. Another project is developing a process and models for measuring cognitive development, particularly executive function and self-regulation, in a child’s first 1,000 days. A separate initiative is designing a technology platform to simulate human organ functions that replaces animal testing in preclinical research. Part of that project aims to extend the technology into restoring organ functions or developing hybrid-synthetic systems to extend failing human organs.
“The last two years,” says CEO Regina Dugan in a Wellcome Leap statement, “have laid bare how much work we have to do in health, equity, and care for the planet. Dugan adds, “We need more breakthroughs to solve the urgent challenges facing the world. And we need them faster.”
Wellcome Leap does not disclose sources of the new $335 million funding. However, Wellcome Trust in January announced plans to raise its charitable spending by £16 billion ($US 21.7 billion) over the next decade, including £750 million ($US 1.02 billion) for large-scale, high-impact projects in the next five years.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 1st, 2022  (Gerd Altmann, Pixabay) 1 Feb. 2022. A developer of cancer diagnostics using genomics to detect precise biomarkers is raising $23 million in the second part of its first venture round. Biofidelity, a three year-old enterprise in Cambridge, U.K., says its process simplifies detection of genetic indicators in tumors enabling more precise therapies for cancer patients.
The Biofidelity technology, called Aspyre, is based on pyrophosphorolysis, a process that reverses conventional chemical synthesis of DNA isolated from samples, with enzymes to directly digest and analyze DNA. For pyrophosphorolysis to work, says Biofidelity, DNA samples must be perfectly matched, otherwise the process is blocked when mismatches occur. Aspyre first isolates and amplifies DNA in samples to enrich target genes for testing. The technology then adds probes with synthetic DNA identifiers representing actionable cancer-causing mutations associated with the target genes.
The probes combine with target regions in patients’ DNA samples indicating a match and the presence of cancerous mutations in the sample, but not mismatched healthy or irrelevant DNA. Matched DNA samples are completely digested, releasing the tail of the DNA probe strand, which forms into a circle. Aspyre seeks out full circular tail formations that indicate complete matches with cancerous biomarkers, while incomplete circles from mismatched DNA are bypassed.
Detect hundreds of biomarkers across multiple genes
Biofidelity says its process is highly sensitive, allowing for detection of single molecules even in small samples from both tissue and liquid biopsies. The process, says the company, can run on current polymerase chain reaction or PCR genomic analysis equipment and return results in under four hours. In addition, says Biofidelity, Aspyre can detect hundreds of biomarkers across multiple genes in a single test without compromising performance.
The company’s lead product is Aspyre-Lung that it says can detect 111 biomarkers — 77 DNA mutations and 34 RNA fusions — across 11 genes, as recommended by international cancer guidelines for non-small cell lung cancer. Biofidelity says the technology can be extended across a broader range of cancer types, extending precision cancer care to more patients.
Biofidelity is raising $23 million in the second part of its first venture round, led by technology investor Octopus Ventures in London, joined by SBI Investment Co. Ltd. and current investors. In Aug. 2020, Biofidelity raised $12 million in the first part of the round, with investments from BlueYard Capital, Longwall Ventures, and Agilent Technologies. At start-up, in Sept. 2019, the company raised $0.75 million in seed funds.
Biofidelity plans to use the proceeds from the round to launch its Aspyre-Lung product. “Our mission,” says Biofidelity CEO Barnaby Balmforth in a company statement, “is to ensure that all patients diagnosed with cancer have access to the genomic information they need to receive the best possible treatment.” Balmforth adds, “This financing is an important step towards making this vision a reality, enabling us to launch our revolutionary technology and to make comprehensive biomarker testing faster, more affordable, and more accessible than ever before.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 31st, 2022  African clawed frog (Brian Gratwicke, Flickr. https://flic.kr/p/dFHy8R) 31 Jan. 2022. Founders of a regenerative medicine company demonstrated a drug delivery device that helps regrow several tissue types on frogs’ amputated limbs. A team from Tufts University in Medford, Massachusetts, led by researchers that started the company Morphoceuticals Inc., describes its findings in the 26 Jan. issue of the journal Science Advances.
The team led by Tufts researchers Michael Levin, professor of regenerative biology, and biomedical engineering professor David Kaplan, are seeking a process that encourages regrowth of multiple types of tissue on amputated limbs. The authors cite data showing 1.6 million Americans living with limb loss in 2005, with that number expected to grow to 3.6 million by 2050. Leading causes of limb amputations are trauma and war wounds, peripheral artery disease, and complications from type 2 diabetes. Among the 2005 population, more than four in 10 amputees (42%) were non-white.
Levin is director of the Allen Discovery Center at Tufts that studies morphogenesis, the process for generating tissue in shapes and patterns, in this case to build and repair complex anatomies. The field, says the Allen Center, includes embryo development, computational biology, artificial intelligence, and signal processing in groups of cells. Kaplan studies regenerative properties of biomaterials, particularly silk, as they interact with stem cells to form new tissue.
The Tufts team designed a process for delivery of several growth compounds to an amputation site to encourage morphogenesis for regrowing multiple tissue types. To demonstrate the process, the researchers used African clawed frogs, a species known formally as Xenopus laevis, that loses its ability to regenerate new tissue in adulthood.
Biodome device worn for 24 hours
The team delivered a collection of five small-molecule growth factor proteins and compounds for controlling adverse reactions, such as inflammation, to the frogs’ hind-leg amputation sites. The drugs were administered through a bioreactor, called a biodome, worn as a 3-D printed sleeve on the site. The biodome is made with a silk hydrogel that releases the regenerative compounds over time. Each frog amputee wore a biodome for 24 hours. The researchers then tracked tissue growth and repair for 18 months.
The authors report by that time treated frogs regrew bone, and repaired nerves and muscles with integrated blood vessels. The treated frogs also restored sensation and motor nerve pathways and began showing formation of digits at the ends of the limbs. In addition, treated frogs also showed movements over surfaces similar to wild-type frogs.
Levin and Kaplan founded Morphoceuticals Inc. in 2020, as a project of Juvenesence Ltd. a company that starts-up, incubates, and invests in enterprises creating therapies for age-related diseases or technology platforms supporting new treatments. In Aug. 2019, Science & Enterprise reported on Juvenesence, based on the Isle of Man in the U.K., raising $100 million in its second venture funding round.
“These findings,” says Juvenesence CEO Greg Bailey in a statement released through BusinessWire, “herald the first application of a new set of tools that will be further developed by Morphoceuticals and will allow us to explore new approaches to regenerative treatment in ways that are truly unique.” Kaplan adds, “These data demonstrate our ability to successfully kickstart endogenous regenerative pathways in vertebrates. However, translation of these findings to mammals remains to be demonstrated as a next key step in this process.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 31st, 2022 – Contributed content –
 (Martin Lopez, Pexels) 31 Jan. 2022. The world is changing and so has science. It is thanks to science that it has been possible to enable the changes that have turned the world into the modern world you live in now. It is through science and the discoveries made that have enabled new technologies to be created that have enabled modern medicine and advancements.
Technologies are advancing and this enables new science to be discovered and new technologies such as gene editing to be explored and decentralized clinical trials are now being offered to ensure the best candidacy, more accurate results and for progress to be made.
Why does science always change?
The scientific community is always evolving. They are challenging and questioning previous results to increase their knowledge and gain clearer understandings.
There is always the opportunity for a new discovery to make. It is through new discoveries that what was previously thought to be correct, can be challenged. Not only can it naturally take effect but the development of science can also be encouraged due to social or political needs.
The discoveries made previously were based on the technologies and knowledge that was once available. As time has progressed and technologies have advanced, knowledge has deepened, more studies have been undertaken. Even if the findings are still the same, scientists now are able to delve into more detail and gain further knowledge. They aren’t just seeing a ‘theory’ they are using their tools to test, prove it and measure it.
Additionally, the ability to share information and data is now more profound. Whereby, once it would have to take a lot of time or geographical obstacles, there are now differing methods available to allow scientists to work together and share ideas and findings.
The ability to share findings, work with other specialists and innovate together, is what brings about development within this constantly changing field.
Why is it important for science to continually change?
As technologies advance and new discoveries are made, new illnesses appear and medical mutations arise, there will always be a need for science to continue to change and adapt.
With all natural processes, it changes and with that so is the need for science to change and develop with it.
In addition to natural progress, there is also the ability to enhance knowledge and challenge previous discoveries. Good science is the ability to take something that was once assumed and accept that the hypothesis was wrong and then explore further their new findings and continually work towards finding out the facts.
It is only inevitable. The more knowledge that is gained the more that is sought. As new scientists take different approaches and views there will always be the opportunity for advancement in this field.
Processes can be streamlined, new evidence will be found, medical breakthroughs will happen. With each new step opening multiple doors for further scientific discoveries to take place.
With these area revolving door of new science and natural changes new scientific research and development will always be needed.
* * *
By Alan, on January 29th, 2022 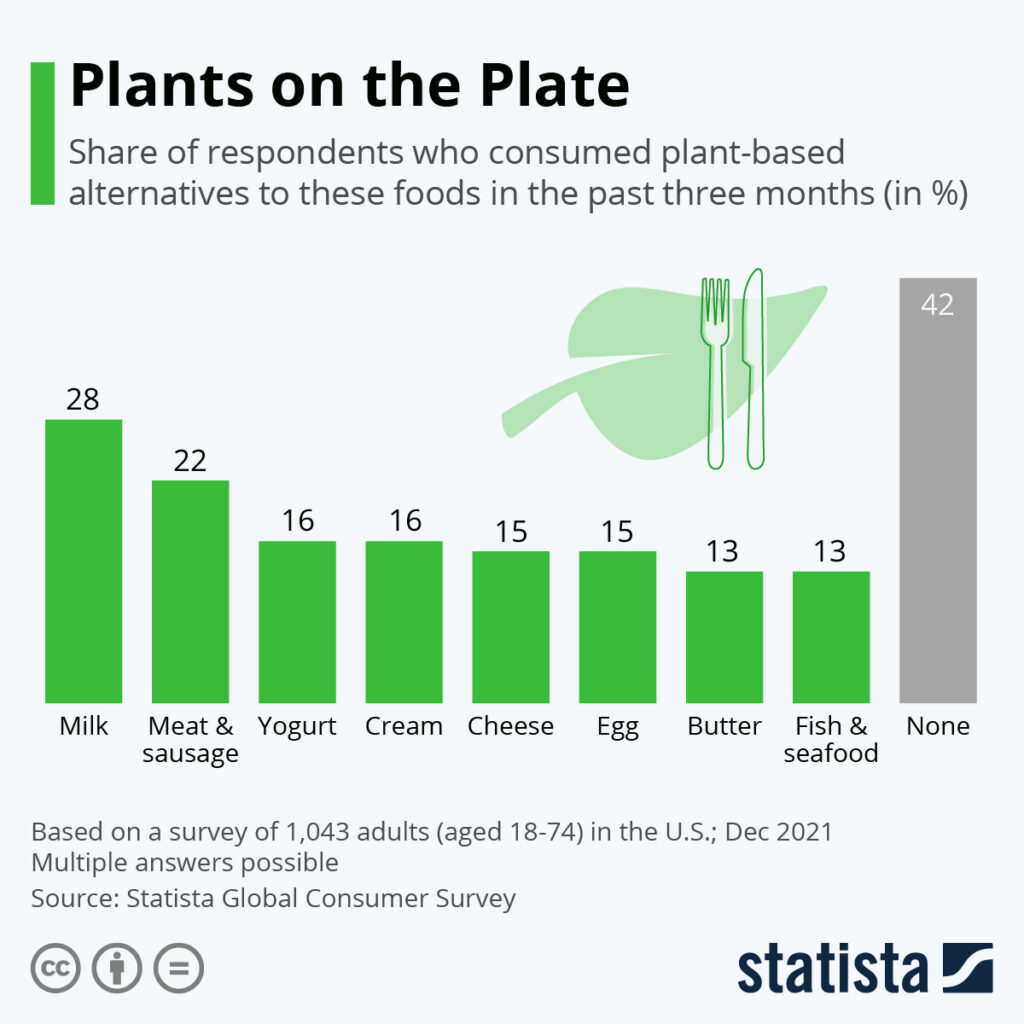 Click on image for full-size view (Statista) 29 Jan. 2022. For the past two years, Science & Enterprise reported on start-up companies developing plant-based alternatives to animal food sources. This week, the business research company Statista posted a chart showing alternative sources for animal food proteins from plants are beginning to gain traction in the U.S.
The data come from a Statista poll as part of its Global Consumer Survey that includes a section on food and nutrition. Statista sampled 1,043 adults in the U.S. in December 2021 asking if they consumed plant-based alternatives to meat, fish, or dairy products in the previous three months. A majority of respondents (58%) said they ate or drank at least one plant-based alternative to meat, fish, or dairy, while 42 percent said they did not.
Not surprisingly, those products on the market longer have more consumers. For example, more than a quarter of respondents (28%) consumed milk alternatives, such as from oats or almonds, while 13 percent said they ate plant-based alternatives to fish or seafood, relatively new to the market. Only about two in 10 (22%) say they tried plant-based meat alternatives, although that number may rise as fast-food outlets add them to their menus.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 28th, 2022  (Fernando Zhiminaicela, Pixabay. https://pixabay.com/illustrations/chemical-molecular-atom-medicine-3086130/) 28 Jan. 2022. A new biotechnology company designing proteins in the lab for difficult drug targets in a range of diseases is raising $100 million in venture funds. Septerna Inc. in South San Francisco is formed and incubated by life science investor Third Rock Ventures, and based on research at Duke University and Monash University in Australia.
Septerna seeks to discover and design small molecule or low molecular weight drugs addressing G protein-coupled receptors, or GPCRs, found on cell surface membranes, and targets for about one in three current prescription drugs, such as antihistamines, ulcer drugs, and beta blockers to treat heart conditions. Yet as the company notes, the vast majority of these drugs today address only six sub-families of GPCRs, leaving out more than 85 percent of potential targets.
Robert Lefkowitz, professor of biochemistry and medicine at Duke University in Durham, North Carolina and co-founder of Septerna, says in a company statement, “the complexity and transmembrane nature of GPCRs have made them difficult to isolate outside of the cell and inaccessible to modern small molecule drug discovery approaches.” Lefkowitz, winner of the Nobel Prize in Chemistry in 2012, is joined as scientific founders by GPCR researchers Arthur Christopoulos, professor of pharmacology, and Patrick Sexton, professor of drug discovery biology, both at Monash University in Melbourne, Australia.
Native functions, structure, and dynamics
Septerna’s Native Complex technology, based on the founders’ research, seeks to recreate the natural world for GPCRs, providing their native functions, structure, and dynamics outside the normal cellular environment. The company says it can isolate, purify, and reconstitute the ligands or binding molecules and supporting signaling proteins found on cell membranes, but outside the cell and in the lab. Septerna says it can screen GPCRs against combinations of ligands and signaling proteins to discover specific receptors previously considered untouchable as drug targets, and with computational techniques and structural biology, design small molecule drugs addressing those receptors.
“Septerna is establishing a new future for GPCR-targeted medicines for patients,” says Septerna CEO and interim president Jeffrey Finer. “We already have strong momentum advancing our pipeline of small molecule drug discovery programs on the path to creating high-impact medicines.” Finer, a Septerna co-founder, is a partner at Third Rock Ventures in San Francisco.
The company is raising $100 million in its first venture funding round, led by Third Rock Ventures. Joining the round are Samsara BioCapital, BVF Partners, Invus Financial Advisors, Catalio Capital Management, Casdin Capital, and Logos Capital. Septerna says the financing will help advance the company’s pipeline across multiple therapeutic areas — endocrine system, central nervous system, inflammation, metabolism — and further develop the Native Complex technology.
Lefkowitz says that technology, “ushers in a new era of drug discovery to reach previously undruggable GPCRs and enable all modern drug discovery technologies to be fully accessible for the GPCR target class.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 27th, 2022  Crispr editing with Cas9 enzyme (Broad Institute, NIH) 27 Jan. 2022. A clinical trial is underway assessing a therapy for HIV infections that aims to eradicate the virus with one dose of the gene-editing technique Crispr. The early- and mid-stage study is testing the treatment code-named EBT-101 developed by the biotechnology company Excision BioTherapeutics Inc. in San Francisco.
Today’s standard of care for HIV infections is antiretorviral therapy, which suppresses and controls, but does not eliminate the virus. Antiretorviral medications must be taken periodically, usually each day, to prevent viral loads from rebounding, since residual levels of HIV remain in infected individuals. Excision Bio says EBT-101, its one-time treatment candidate, is a functional cure for HIV.
According to Centers for Disease Control and Prevention, 36,801 Americans were diagnosed with HIV infections in 2019, a decrease of nine percent from 2015. About seven in 10 (69%) of those new cases were gay or bisexual men, with nearly a quarter (23%) heterosexual, and seven percent people who inject drugs. Worldwide, according to World Health Organization, nearly 38 million people were living with HIV in 2020, with 1.5 million new cases reported that year.
Excision Bio uses gene editing to remove large sections of DNA from HIV viruses, taking out the viruses’ ability to replicate and escape. For these edits, the company adapts Crispr, short for clustered regularly interspaced short palindromic repeats, a process based on bacterial defense mechanisms that use RNA to identify, monitor, and edit targeted locations in DNA. Excision says its Crispr process employs dual guided RNA probes with Cas 9 enzymes to eradicate parts of the viral DNA responsible for reproduction and viral escape. The result, says the company, is one EBT-101 treatment can disable HIV-1 viruses, the more common and infectious strain, preventing their replication and thus stopping further infection.
Safety and tolerability of Crispr
The clinical trial is enrolling nine male adults with HIV-1 infections and on an antiretroviral regimen, but with viral quantities below quantifiable levels. Participants at the four sites in the U.S. are randomly assigned to receive one of three dose levels of EBT-101, delivered with benign adeno-associated viruses often used in gene therapies. The study team is looking primarily for signs of adverse events in recipients in the next 48 weeks, using a standard grading guide for clinical trials of this kind.
Rachel Presti, lead investigator of the trial and professor of infectious diseases at Washington University in St. Louis, says in a company statement released through Globe Newswire, “We’ll be looking at the safety and tolerability of Crispr in removing fragments of viral DNA from the genome of infected cells and tissues that are known to be reservoirs of HIV. The existence of such reservoirs has been a major hurdle in our efforts to cure HIV.”
The team is also tracking efficacy indicators, including activity of the treatments in patients. Science & Enterprise reported in Sept. 2021 on the Food and Drug Administration accepting Excision Bio’s investigational new drug application for EBT-101, clearing the way for the trial.
Lisa Danzig, Excision Bio’s chief medical officer notes, “Forty years into this pandemic there is still no curative regimen,” adding “we are now working to validate our preclinical findings in the clinic by generating safety, pharmacodynamic, and preliminary efficacy data. We believe these data will inform the design of future studies and demonstrate EBT-101’s potential to address the unmet needs of patients with HIV.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 26th, 2022  (Nadine Doerlé, Pixabay. https://pixabay.com/photos/child-tablet-technology-computer-1183465/) 26 Jan. 2021. A creator of game therapies for cognitive impairments is becoming a publicly-traded company through a special purpose acquisition company or SPAC. The merger with Social Capital Suvretta Holdings Corp. is expected to bring shareholders in Akili Interactive Labs Inc. up to $412 million in cash, with the company’s market value estimated at $1 billion.
Akili Interactive develops computer games to treat cognitive deficiencies that the company says is based on neuroscience and algorithms to deliver personalized physiological changes in the brain. Akili says its technology is designed for disorders in regions of the brain affecting attention and focus, multi-tasking, memory and goal management, spatial navigation, planning, and organization.
The company’s first product is EndeavorRx, a computer game therapy for ADHD, short for attention deficit hyperactivity disorder. ADHD is a condition in children marked by difficulty paying attention, staying focused, controlling behavior, and high levels of activity. Centers for Disease Control and Prevention estimates ADHD affects some 6.1 million children in the U.S. age 2 to 17. Current treatments for ADHD, says the company, include medication and behavioral therapies, which are limited by undesired side effects from medications, and a shortage of trained therapists, as well as varying insurance coverage for therapies.
Akili says EndeavorRx delivers sensory and motor stimuli to the prefrontal cortex, the part of the brain dealing with attention and other cognitive functions, such as memory. The company says the game is designed to give children with ADHD a fun experience while stimulating and exercising these brain centers to improve multitasking, focusing on goals, and avoiding distractions. The game is guided by algorithms that personalize the treatments to patients’ specific conditions and progress.
A new approach to cognitive science
Science & Enterprise reported in June 2020 on FDA’s authorization of EndeavorRx for use with a therapy, medication, or education program guided by a clinician. Akili says the game also received a CE mark, clearing EndeavorRx for use in Europe. The company says it’s developing interactive game therapies for other neurological conditions: multiple sclerosis, major depression, autism spectrum disorder, and Covid-19 brain fog.
Akili Interactive is going public by merging with Social Capital Suvretta Holdings, a SPAC enterprise formed as a joint venture between private equity companies Suvretta Capital Management and Social Capital. Social Capital Suvretta Holdings is already a public company, listed as DNAA on the Nasdaq exchange, with the aim of acquiring a biotechnology company, in this case Akili Interactive.
Chamath Palihapitiya, chairman and CEO of Social Capital Suvretta notes in an Akili Interactive statement, “Akili is taking a new approach to cognitive science, using software to target our underlying cognitive function and creating an entirely new class of medicine as a byproduct.” Palihapitiya adds, “Akili has the unique opportunity to change how we treat pediatric ADHD. They have also laid the groundwork to treat a wide range of other cognitive issues affecting tens of millions of people around the world.”
The merger is expected to complete in mid-2022, with current Akili investors receiving up to $412 million in cash. Of that total, $250 million is provided by Social Capital Suvretta and another $162 million raised from a private investment in public equity, or PIPE transaction, a private means of raising capital for public-company acquisitions at a reduced share price, in this case $10.00 per share. When the transaction is complete, Akili Interactive will trade on the Nasdaq exchange under the ticker symbol AKLI, and is expected have a market capitalization of $1 billion.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 25th, 2022  (Elchinator, Pixabay) 25 Jan. 2022. A provider of artificial intelligence services for scientific and advanced technology applications is raising $100 million in its second venture round. InstaDeep Ltd. in London creates deep learning systems, a type of A.I., for a range of industries, including biotechnology companies developing vaccines and diagnostics for Covid-19 infections.
Deep learning is a form of A.I. that makes it possible for systems to discern underlying patterns in relationships, and build those relationships into knowledge bases applied to a number of disciplines. In deep learning, neural networks that emulate brain functions revise those algorithms or models with new data added to the store of knowledge. The algorithms include perceptrons or classifiers to weight the value of new data added to the knowledge base during the learning process.
InstaDeep develops A.I. solutions for different business sectors including biotechnology enterprises. The company offers mathematical models for simulating complex protein interactions called DeepChain for designing biologics and diagnostics. One of InstaDeep’s customers is BioNTech SE in Mainz, Germany, developer of a leading Covid-19 vaccine using messenger RNA to invoke an immune reactions against the SARS-CoV-2 virus.
A.I.-assisted circuit board design
As reported in Science & Enterprise earlier this month, InstaDeep is also collaborating with BioNTech on an early-warning public health system to quickly detect dangerous SARS-CoV-2 variants. In a test of the system, posted in a preprint or non-peer reviewed paper, the InstaDeep/BioNTech team identified in minutes 12 of the 13 variants flagged as dangerous by World Health Organization. The authors say an early warning system employing those models would have spotted these mutations an average of two months sooner. That includes the omicron variant, which the authors say they detected the same day its sequence became publicly available.
InstaDeep also offers a routing design service for printed circuit board providers, now in beta version. The service called DeepPCB accepts uploaded schematic files with components on the board and returns a routing for connections among layers and components within 24 hours. The company says manual board routing can take weeks, although other commercial routing software is available. In addition, InstaDeep says it develops A.I. solutions for companies in logistics, manufacturing, energy, and mobility/ride-sharing services.
InstaDeep is an eight year-old company with offices in Tunis, its first location, and Paris, Lagos, Dubai, and Cape Town. The company is raising $100 million in its second venture round led by Alpha Intelligence Capital in Luxembourg and CDIB Capital in Hong Kong. Also taking part in the round are BioNTech, Chimera Abu Dhabi, Deutsche Bahn’s DB Digital Ventures, Google, G42, and Synergie. Google, Deutsche Bahn (Germany’s railroad system ), and as noted above BioNTech, are collaborators with InstaDeep. According to Crunchbase, the company raised $7 million in its first funding round in May 2019.
Arnaud Barthelemy, partner at Alpha Intelligence Capital, says in an InstaDeep statement, “InstaDeep is a deep technology company that disrupts traditional approaches in crucial areas as they do with BioNTech for drug discovery or with Deutsche Bahn for railway scheduling.” The company plans to use the new funds to advance its computing infrastructure, hire new talent, launch new products across multiple industries, and expand into the U.S. market.
More from Science & Enterprise:
Disclaimer: The author owns shares in Pfizer, BioNTech’s partner in Covid-19 vaccines.
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on January 24th, 2022  (Rawpixel, Unsplash) 24 Jan. 2022. A new not-for-profit organization aims to create a more efficient process for large-scale randomized clinical trials, to provide reliable results at lower cost. Protas, founded last year in London, announced a £5 million ($US 6.7 million) grant from Paris-based drug maker Sanofi, to supplement the group’s initial funding from the U.K.’s National Health Service or NHS.
Protas plans to work with industry and academic researchers, health care providers, patient organizations, and medical foundations to design large-scale clinical trials testing new treatments for a wide range of diseases. The organization is developing a collaborative process for clinical trials that includes advances in technology to make trials more efficient and inclusive, while delivering reliable findings.
The work of Protas is based in part on the experience of its founder Martin Landray, professor of population health at University of Oxford, who leads the Randomized Evaluation of Covid-19 Therapy or Recovery trial testing a large number of treatments for people with Covid-19 disease. The Recovery trial is enrolling 50,000 participants in the U.K. and four other countries to test a wide range of drugs to treat Covid-19, from simple aspirin to new synthetic monoclonal antibodies. Among the trial’s findings, says Protas, is the efficacy of dexamethasone, a common inexpensive steroid drug, to treat severe Covid-19 cases.
The organization seeks to deliver clinical trials with a common standards-based information technology platform, while also tailoring its studies to meet specific, scientific, regulatory, and patient-care needs. Protas says trials will be designed for the full process life-cycle of clinical studies from planning and recruitment to delivery of results for sponsors and regulators.
Prospect of fewer treatments in the pipeline
As an example, the Recovery trial uses an adaptive design that allows for adding or removing different treatment candidates or population groups, using a common study protocol and streamlined consent forms. In addition, the Recovery trial, seeks access to patients’ electronic health records for follow-up, as well as conventional in-person or online data collection.
Protas plans to apply lessons learned from the Recovery trial to a broad collection of diseases. “The situation is not
[unique] to the pandemic,” says Landray in a Protas statement. “there are many other common and other life-threatening diseases — for example heart, lung and respiratory disease, arthritis, cancer, depression, and dementia — where better treatments are needed to reduce the huge burden on patients and the NHS.”
But developing new therapies faces increasingly higher costs, thus the need for a new process to better control those costs or the prospect of fewer treatments in the pipeline. Dietmar Berger, chief medical officer at Sanofi, says the need for lower clinical trial costs influenced the company’s decision to become a Protas partner. “With this collaboration,” says Berger, “we are taking a bold [step] to significantly reduce the cost of some of our clinical trials, focusing on what matters the most for patients, doctors, regulators, and payers.”
Protas plans in 2022 to continue building its organization and add partners, with the first clinical trials expected next year.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|










 RSS - Posts
RSS - Posts
You must be logged in to post a comment.