Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on February 12th, 2022 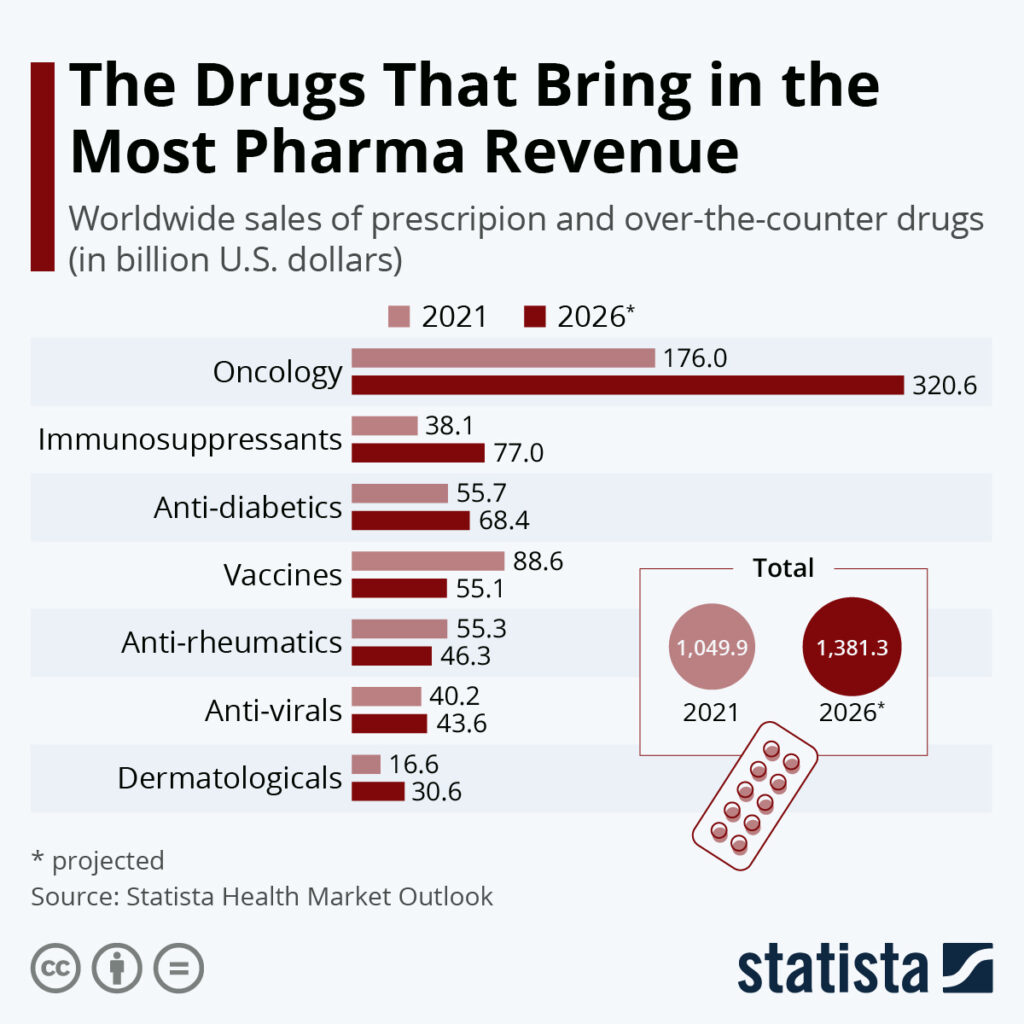 Click on image for full-size view (Statista) 12 Feb. 2022. In December, we ran a chart showing vaccines make up a small fraction of overall drug sales worldwide, even Covid-19 vaccines. Our infographic this weekend broadens the issue to revenue sources overall for drug makers, and the leader by a wide margin is cancer drugs.
The infographic’s data are compiled by business research company Statista in its Health Market Outlook, a paid report, and displayed in a chart earlier this month. Statista’s data show global cancer drug sales brought in $176 billion in 2021, 17 percent of total pharmaceutical industry revenues of $1.05 trillion. Vaccine sales totaled nearly $89 billion last year, with diabetes and rheumatic drugs for arthritis and other inflammatory conditions each selling $55 to $56 billion.
By 2026, however, cancer drug sales are expected to grow to nearly $321 billion, making up 23 percent of all pharma revenues. Also by 2026, vaccine sales are projected to fall to $55 billion, but immunosuppressant drugs — biologics that limit the immune system — are expected to grow to $77 billion and take over second place. Pharma companies gained about $40 billion from anti-viral drugs in 2021, but that sales volume is not expected to grow much, reaching less than $44 billion in 2026.
Statista compiled the data from business, consumer, and government spending on pharmaceuticals, with projections based on models composed of financial, health care, and economic variables.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 11th, 2022  (MaxPixel. https://www.maxpixel.net/Cream-Pharmaceutical-Ointment-Gel-Skincare-1243604) 11 Feb. 2022. A drug maker acquired commercialization rights to an injectable hydrogel developed at a university lab that delivers proteins to repair diseased tissue. Alkem Laboratories Ltd. in Mumbai, India is licensing the hydrogel technology created in the lab of David Mooney, professor of biomedical engineering and a researcher at the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Mooney and colleagues study cell and molecular biology with materials science, to better understand therapeutic signals that can be transmitted through bio-based materials. Among the areas investigated in the lab is tissue engineering, particularly repair or regeneration of tissue damaged by disease or trauma. In some cases, regenerating or repairing damaged tissue is difficult with systemic treatments, such pills, capsules, or injections, where high doses could harm the patient. Thus, direct and sustained delivery of a treatment to affected sites is preferred.
One of the materials studied in Mooney’s lab is hydrogels, water-based gels structured with biocompatible polymers. In this case, hydrogels provide enough of a framework to deliver therapeutic molecules, yet can still be injected with a conventional syringe. “Scientists can point to many promising treatments for diseases and injuries that have never made it to the clinic,” says Mooney in a university statement, “not because they don’t work, but because delivering them via a classic injection or pill wasn’t possible.”
Provide safe doses of protein therapies
In Sept. 2019, researchers in Mooney’s lab published results of study of muscle and nerve regeneration with lab mice and rabbits, using a hydrogel with alginate, a natural material derived from seaweed and used as a food thickener. The hydrogel delivered concentrations of therapeutic proteins — vascular endothelial growth factor or VEGF, and insulin-like growth factor-1 or IGF-1 — injected under the skin. In mice, the protein-laden hydrogel helped repair and improve functioning of sciatic nerves, and in rabbits the treatment helped speed healing of transplanted facial muscles.
Alkem Laboratories produces generic and specialty pharmaceuticals sold in India and worldwide. The company’s products include treatments for skin diseases, complications from diabetes, and nerve disorders. In this deal, Alkem is licensing the Mooney lab’s hydrogel technology with a chemical framework for delivering therapeutic proteins, such as VEGF or IGF-1 growth factors. The hydrogel is expected to provide safe doses of protein therapies for sustained release to treat conditions such as nerve damage and foot ulcers resulting from diabetes, and vascular disorders such as peripheral artery disease. The agreement gives Alkem Labs commercialization rights for the hydrogel in India and the U.S.; financial terms were not disclosed.
“This technology’s novel, regenerative medicine approach,” notes Alkem Labs president Akhilesh Sharma, “could help fill a therapy gap in the treatment of multiple causes of ischemic tissue injuries, with the potential to avoid several thousands of foot deformities and amputations and provide relief from other ischemic conditions.”
This deal is the second licensing agreement from Wyss Institute revealed this week, and reported by Science & Enterprise. On Tuesday, the institute announced a deal with a start-up company Trestle Therapeutics for rights to a stem cell and three-dimensional printing process to produce tissue needed by people with kidney failure. Also, in July 2021, a Florida company gained rights to to develop dental sutures and membranes from an alginate adhesive gel developed in Mooney’s lab.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 10th, 2022  (Arek Socha, Pixabay) 10 Feb. 2022. The Food and Drug Administration released for comment review processes for new non-opioid drugs to relieve acute or temporary pain episodes. The draft regulatory guidance aims to provide drug makers with the agency’s latest thinking on development, product labeling, and expedited review programs for non-opioid pain relief drugs.
FDA’s document defines acute pain as “pain, lasting up to 30 days, typically in response to some form of tissue injury, such as trauma or surgery.” Opioid drugs are still prescribed for acute pain in many cases, which can lead to dependence or abuse. In the U.S., abuse of opioid pain drugs continues at rates reaching emergency levels, along with heroin and fentanyl sold on the street. Overdose deaths from opioids, according to National Institute on Drug Abuse, reached nearly 50,000 in 2019, with the economic burden from abuse of prescription pain drugs totaling $78.5 billion a year. In addition, a 2018 law calls for FDA to develop regulatory guidance addressing the need for non-opioid pain drugs.
“Preventing new addiction through fostering the development of novel non-opioid analgesics is an important priority for the FDA,” says Patrizia Cavazzoni, director of the Center for Drug Evaluation and Research, in an FDA statement. “The guidance reinforces the agency’s commitment to confront opioid misuse, abuse, and addiction by taking steps to help those with acute pain get access to improved non-opioid treatment alternatives.”
The document spells out features of clinical trials sought by FDA for testing non-opioid pain relief drug candidates. In general, FDA calls for randomized, double-blind, superiority trials, testing repeated doses of a new drug candidates for at least 24 hours. Primary endpoints or main target medical outcomes of the trials, says the document, should be changes in measures of pain intensity, such as sum of pain intensity difference, for the duration of the treatment, tested against a placebo or other pain relief drug.
Measure reductions in opioid use
FDA’s proposed guidance recommends taking frequent pain intensity measures and pre-defined time points, e.g. every hour or four hours over a specified period, beginning as close as possible to the start of a pain episode, such as immediately after surgery. The document also calls for pre-specifying rescue medications designed to quickly relieve pain. Trial protocols, says the agency, need to spell out frequency, quantity, and pain thresholds allowed for rescue pain relievers, since use of rescue drugs in comparison groups could diminish the measured effects of test drugs.
FDA’s discussion of endpoints also covers reduction in opioid use by patients. The document says clinical trial protocols may note quantified reductions in numbers of doses overall or per day, or fewer days using opioids. Endpoint outcomes may include as well reductions in adverse effects from reduced opioid use. Measuring safety of new non-opioid drugs in trials, says the guidance, will depend on novelty of the drug’s mechanism, with new types of pain drugs needing to show more evidence than extensions of existing drugs.
FDA’s guidance spells out different claims drug developers can make about new pain drugs reducing use of opioids: eliminating use of opioids for pain relief, discharge from medical facilities without opioids, and reduction of adverse effects associated with opioids. In addition, the document gives examples of data needed to back up those claims, and encourages follow-up studies that track longer-term effects, including extended benefits of non-opioid pain relief.
The draft guidance spells out FDA programs for expedited review that could apply to non-opioid pain drug candidates. The document notes, however, that pain intensity is a subjective measure provided only by the patient, thus surrogate endpoints often used in expedited reviews might not fit as well for these drugs. But reduced abuse potential of a proposed non-opioid pain drug may be considered if applicable.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 9th, 2022  (Seanbatty, Pixabay) 9 Feb. 2022. Three venture capital investors specializing in robotics, artificial intelligence, and other advanced technologies raised $365 million for start-up financing. Morpheus Ventures in Los Angeles, Omega Venture Partners in Palo Alto, and Cortical Ventures in San Francisco and Boston announced new investment funds of $200 million, $115 million, and $50 million respectively.
Morpheus Ventures invests in early-stage technology enterprises providing investment capital, and according to the company, help for new businesses to get off the ground and through complexities and hazards of the technology industry. That assistance, says Morpheus, can include recruiting key executives, making connections with customer prospects, and protecting intellectual property. Morpheus’s portfolio covers companies in data analytics, artificial intelligence, robotics, consumer and business-management technologies, and software as a service.
Morpheus raised $200 million for its second discretionary capital fund it calls Fund II. The company plans to use Fund II to finance businesses working in financial, insurance, and property management technologies, as well as companies exploring robotics and other advanced disciplines. In January, Science & Enterprise reported on VeriSIM Life in San Francisco a company using machine learning algorithms to develop new drugs designed to better succeed in clinical trials that raised $15 million in its first venture round, led by Morpheus Ventures.
“With this closing,” says Morpheus managing partner Joseph Miller in a company statement released through Cision, “the new fund takes the total committed capital across all Morpheus funds and vehicles to over $300 million, making us one of the largest early-stage VC funds in Los Angeles.”
Most lucrative and exciting secular trend
Omega Venture Partners is also an investor in early-stage businesses, particularly companies developing artificial intelligence applications. The company says it invests in new businesses developing A.I. software applied to solving high-value problems and opening new revenue streams. Omega Venture says its optimum investment targets are companies that go beyond automating routine tasks, but also enabling creativity, increasing empathy, and improving judgement. The company says it connects its portfolio companies to a network of experienced business and technology executives and experts.
Omega Venture says it raised $115 million for its new fund, exceeding the original $100 million target. “The application of artificial intelligence to deliver breakthrough business solutions and to unleash vast revenue growth opportunities,” says Omega Venture managing partner Gaurav Tewari in a company statement released through Cision, “is the most lucrative and exciting secular trend I have seen in my 16-plus years of leading venture capital firms.”
Cortical Ventures is another investor in early-stage businesses building artificial intelligence systems or services, that began its public operations today. The company aims to be both an investor, funding seed and early venture rounds, but also an incubator for A.I. start-ups. And Cortical Ventures says it’s developing an A.I. application of its own for managing relationships with investors and portfolio companies.
Cortical Ventures raised $50 million in its first investment fund. The company is founded by data scientist Jeremy Achin, who founded A.I. services provider DataRobot in 2012. Achin, now Cortical Ventures’ general partner, says in a statement released through Cision, “our inaugural early stage fund as a first step to building a generational A.I. investment franchise and to partner with the best entrepreneurs.”
The company announced participation in two A.I. seed stage venture rounds, with both companies founded by Achin, and currently operating in stealth mode in Boston. Neo Cybernetica is raising $30 million in seed funds, while hOS is raising $12.8 million. Both seed rounds are led by technology investor New Enterprise Associates in Menlo Park, California, with Cortical Ventures and other investors taking part.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 9th, 2022 – Contributed content –
 (Malachi Witt, Pixabay) 9 Feb. 2022. Growth is one way to survive and establish yourself as a credible brand, no matter your industry. You can do this by gaining enough skills and knowledge that will help you advance in your career. In 2020, the investments made into leadership training alone were valued at $357.7 billion worldwide, indicating that it is beneficial. Consider these practical tips for stepping things up when it comes to your reputation.
1. Build your network
Even though you are likely to be in competition with some players in your industry, you can still build some alliances. Even after that, it will be helpful to stay in touch by participating in industry events and activities. For example, if you are a researcher, it will be helpful to review your peers’ work. This portrays you as knowledgeable, and if you are on platforms like Nova Science Publishers review, you will get credit for your work. This is a great way to be a part of a community you can rely on in times of a crisis. In a world where reputation means a lot, having people that can vouch for you is a good strategy.
2. Stick to your word
Being reliable and dependable is a sure way for people to trust you, and you can start by doing what you say you’ll do. Always following true with your word tells people that they can trust you to get the work done. This may seem like a simple thing. Sometimes, you may be swarmed and so you almost forget some things. What you can do is master time management, use a schedule, or delegate some tasks to others. This will help you make time for everything without burning out.
3. Look the part
In the ideal world, people should not be judged or treated according to how they look outside. Unfortunately, it is not the case, and to some extent, there is some validity to it, as in some cases, people who look credible are. Therefore, make sure that you are giving an impression that projects trustworthiness. For example, ensure you are always wearing clean clothes appropriate for the event you are attending. If you operate a website, ensure that it looks professional, with impeccable spelling and colors that do not hurt the eyes. If you are on social media, your feed and posts should reflect your industry knowledge. The goal is to always be impressive.
4. Always go the extra mile
What differentiates extraordinary from the average is your ability to go the extra mile. Many people always appreciate the effort and are likely to consider your brand as a reputable one if you always do more than expected. They will be more willing to come to you because they will be getting more value, which is a good way to stay ahead.
Building a reputation for yourself in your industry opens doors for you in many ways. These are a few tips you can use to achieve that.
* * *
By Alan, on February 8th, 2022 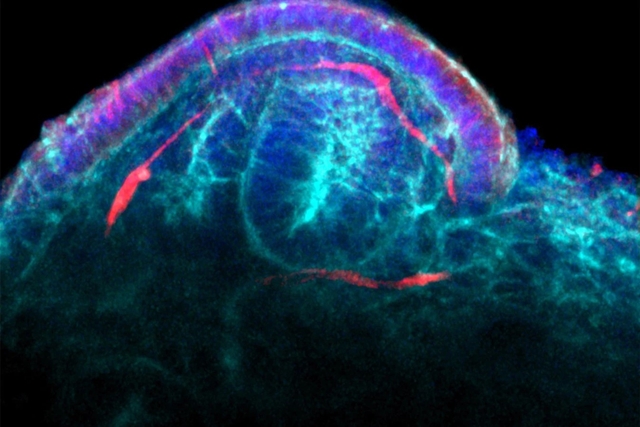 A capillary developing in a glomerulus in a kidney organoid. (Wyss Institute, Harvard University) 8 Feb. 2022. A start-up company is acquiring the rights to a stem cell and three-dimensional printing process to produce tissue needed by people with kidney failure. Trestle Biotherapeutics Inc. in La Jolla, California is licensing for commercialization, technologies created in the biomedical engineering and materials science labs of Jennifer Lewis and Ryuji Morizane at Harvard University’s Wyss Institute for Biologically Inspired Engineering.
Trestle Biotherapeutics is a two year-old enterprise developing engineered tissue for patients with end-stage renal disease, a life-threatening condition where the kidneys almost completely fail. For people with this disorder, the only treatment options are frequent dialysis sessions to clean impurities from the blood, or a transplanted kidney with donors in short supply. According to the Kidney Project, some 750,000 people in the U.S. and 2 million people worldwide have end-stage renal disease.
The company cites data showing in 2021 more than 100,000 patients are waiting for a kidney transplant, and more than 550,000 patients rely on dialysis for survival. “Patients living with kidney failure have had the same two standard-of-care treatment options for more than 60 years,” says Trestle Bio co-founder and CEO Ben Shepherd in a statement released through BusinessWire.
3-D printed kidney organoids
Among the Wyss Institute’s research initiatives is production of engineered tissue and organs with 3-D printing. One of this project’s goals is to replicate as much of the complexity as possible in human organs, including blood vessels, with 3-D printers. Lewis’s lab at Wyss Institute and the university’s engineering school studies 3-D bioprinting to produce multiple cell and tissue types, that include blood vessels. Morizane’s lab at Mass. General Hospital in Boston, affiliated with Wyss Institute and Harvard’s Stem Cell Institute, studies stem cells for regenerating functioning kidney tissue. In Feb. 2019, Science & Enterprise reported on development of 3-D printed kidney tissue chips called organoids with blood vessel networks produced by Lewis, Morizane, and colleagues.
Trestle Bio says the technology licensed from Harvard makes it possible to produce 3-D printed kidney tissue with blood vessels intact in quantities needed for regenerative medicine. The process also enables tissue to mature and further develop blood vessels within organoids, which the company says can be the basis for larger functioning tissue transplants in kidney failure patients. Lewis and Morizane are joining Trestle Bio as scientific advisors. Financial terms of the licensing agreement are not disclosed.
“Trestle was founded with the belief that recreating patterns and processes found in nature is key to building functional tissues,” says Alice Chen company co-founder and chief scientist. “The next era of cell therapies and regenerative medicine, particularly for addressing diseases arising from complex organs such as the kidney, will rely on the integration of multiple advancing disciplines. Developmental biology, stem cell biology, and 3D biofabrication are core components of this approach.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 7th, 2022  (Gerd Altmann, Pixabay) 7 Feb. 2022. Two biotechnology companies created a synthetic non-infectious target model to represent SARS-CoV-2 viral variants for vaccine and therapy developers. A team from Twist Bioscience Corp. in San Francisco and Eleven Therapeutics in Cambridge, U.K. describes its synthetic RNA model called a replicon, in a paper posted Saturday on bioRxiv, a pre-publication repository for biomedical journal articles not yet peer-reviewed.
A replicon is an engineered virus with infection-causing components removed. The remaining parts of the virus provide enough of the viral genome as a target for researchers to develop new vaccines or therapies. Replicons are already in use to mimic Zika, dengue, and SARS-CoV-1 viruses. In this project, researchers are seeking a replicon target for the SARS-CoV-2 virus responsible for Covid-19 with the ability to replicate, but still not infect cells. In addition, the design technology needs to produce replicons that accurately represent different SARS-CoV-2 variants already circulating, as well as those yet to emerge.
Twist Biosciences develops synthetic genetic materials on a silicon platform, patterned after semiconductors, instead of traditional plastic plates and receptacles. This process, says the company, overcomes conventional limitations and inefficiencies to design and construct genes, and from these synthetic genes, produce libraries of genetic variations. As reported by Science & Enterprise in July 2021, Twist Bio used its technology to design genetic reference materials for tests that detect the then-quickly spreading SARS-CoV-2 delta variant.
Eleven Therapeutics applies computational techniques, including artificial intelligence, to biochemistry. The company says its technology uses massive parallel synthesis to produce programmable small synthetic RNA molecules that can silence or interfere with disease-causing targets, with a current pipeline focusing on respiratory diseases.
Introduce and test small genomic alterations
In their paper, researchers from both companies led by Eleven Therapeutics CEO Yaniv Erlich, describe a process for creating replicons with certain needed properties, based on published genetic sequences. As a result, says the team, this process allows vaccine and therapy developers to use synthetic replicons as design targets without transferring live viral material. In addition to reducing safety concerns, labs do not need to prepare material transfer agreements that can add significant bureaucratic delays at a time when speed is essential.
The Twist/Eleven process first screens for all available genetic sequencing data to create a consensus sequence for a particular viral strain or variant. The system then uses algorithms to introduce and test a series of small simulated alterations to the sequence to find an optimal sequence that removes all ability of the virus to infect cells, but retains other viral properties.
To prove the concept, the study team designed a replicon simulating the SARS-CoV-2 beta variant, previously named B.1.351, and first discovered in South Africa in May 2020. Tests in secure biosafety labs, say the authors, show the replicon is non-infectious. Further tests with the authorized Covid-19 antiviral drug molnupiravir and Eleven’s RNA-interference candidates show the replicon responds to treatments like the live virus.
In a Twist Biosciences statement, Erlich says the replicon technology can be readily and quickly applied to other SARS-CoV-2 variants. “We developed this in only three weeks,” says Erlich, “showing that both the active ingredient in the commercial antiviral drug molnupiravir and our proprietary RNAi treatment, which had already been known to effectively treat other variants of the SARS-CoV-2 in live virus samples, inhibited the replicon effectively, confirming therapeutic development applicability for the replicon.”
“Replicons offer a safe alternative to study viruses and represent another potentially important tool in fighting SARS-CoV-2,” notes Emily Leproust, CEO of Twist Biosciences. Leproust adds, “Using the framework we developed, researchers can quickly establish new assays to develop specific therapeutics and study variant characteristics.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 5th, 2022 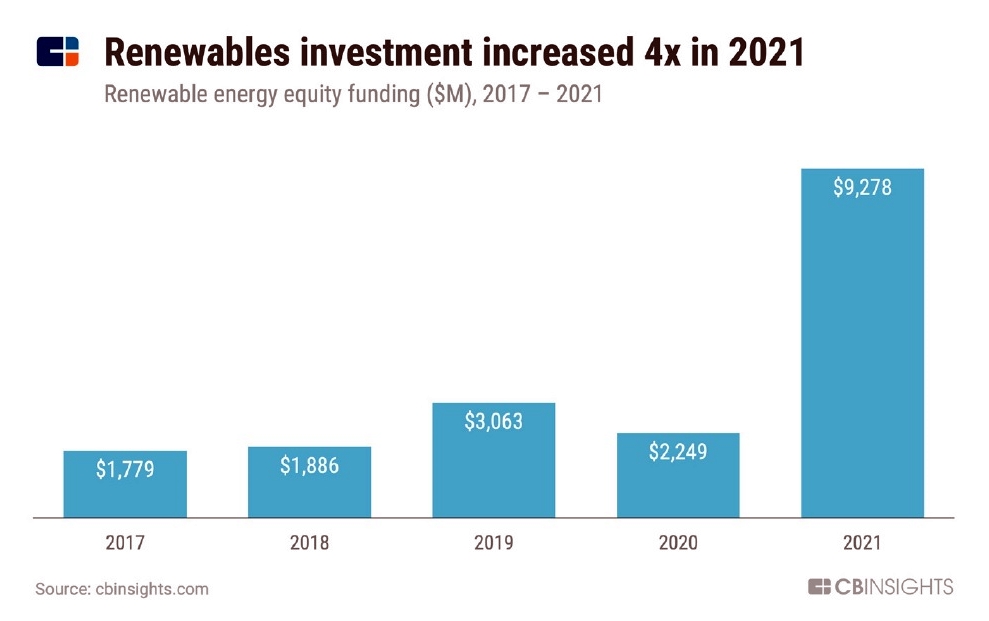 Click on image for full-size view (CB Insights) 5 Feb. 2022. While governments inched forward in November on a global climate agreement, private investors are not waiting to make substantial commitments to battle the climate crisis. Technology research company CB Insights shows in its recent Tech Trends for 2022 report (registration required) that venture investments in 2021 more than quadrupled from 2020 to nearly $9.3 billion, in companies developing technologies addressing the climate crisis.
CB Insights defines climate technology investments as funding for companies working in energy storage, hydrogen, carbon capture, vertical farming, electric vehicles, smart buildings, and alternative food protein sources to conventional farming. In 2020, those companies raised $2.25 billion, a decline from a little more than $3 billion raised in 2019. In 2021, however, climate technology investments zoomed to $9.28 billion.
In addition, says CB Insights, venture funds dedicated to climate technologies reported attracting plenty of investors. Lowercarbon Capital in New York, according to Crunchbase, raised $800 million for its first fund in Aug. 2021. And, says CB Insights, two European venture investors, World Fund and 2150, raised $400 million and $300 million respectively last year. But CB Insights also notes that climate technologies face a particular challenge in demonstrating economic payoffs for their products, and often need intensive capital infusions that delay returns to investors.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 4th, 2022  (Gordon Johnson, Pixabay) 4 Feb. 2022. In tests with lab animals, an experimental vaccine is shown to generate antibodies responding to toxic brain proteins associated with Alzheimer’s disease. Findings from the study appear in Wednesday’s issue of the journal Brain Communications, assessing the vaccine code-named ACI-24 by its developer AC Immune SA in Lausanne, Switzerland.
AC Immune creates diagnostics, vaccines, and treatments for Alzheimer’s disease and other neurodegenerative disorders. Alzheimer’s disease is a progressive neurodegenerative condition, the most common form of dementia affecting growing numbers of older people worldwide. People with Alzheimer’s disease often have deposits of abnormal substances in spaces between brain cells, known as amyloid-beta proteins, as well as misfolded tangles of proteins inside brain cells known as tau. Data from Alzheimer’s Disease International show in 2020 more than 55 million people worldwide with dementia, at least half of those with Alzheimer’s disease, a number expected to grow to 139 million by 2050.
AC Immune uses two separate technologies to address toxic protein deposits, both amyloid-beta proteins that accumulate as plaques outside of neurons and tau that builds up inside neurons in the brain and spreads between cells. One of those technologies, called SupraAntigen, is the basis for treatments that generate antibodies in the immune system for attacking and breaking up toxic protein deposits, particularly before they cause irreversible damage to neurons.
High concentrations of antibodies
In this study, researchers from AC Immune tested ACI-24, a vaccine for preventing Alzheimer’s disease and treating the disorder in its early stages, in lab mice and monkeys. ACI-24, says the company, is produced with SupraAntigen as a synthetic peptide, or short chain of amino acids, with an adjuvant to enhance immune response, and carried in a lipid or natural oil container. In this case, the peptide in ACI-24 is designed to invoke an immune reaction, with antibodies to clear a toxic amyloid-beta protein known as pyroglutamate abeta3-42 or pGlu-Abeta3-42. And those generated antibodies specifically target 15 amino acids making up a key part of pGlu-Abeta3-42.
The study team says test animals well-tolerate ACI-24. Recipients of ACI-24, mice and monkeys induced with pGlu-Abeta3-42, produce high concentrations of immunoglobulin G antibodies that react with the toxic protein deposits. The authors say the antibodies generated by ACI-24 cover a broader range of binding sites on target proteins than earlier immunotherapy candidates for Alzheimer’s disease targeting amyloid-beta, or Abeta.
AC Immune is developing ACI-24 as a treatment for individuals in early or mild stages of Alzheimer’s disease. In this study, the company used an optimized formulation of ACI-24. An earlier form of the vaccine is in early- and mid-stage clinical trials, evaluating ACI-24 among Alzheimer’s disease, or AD, patients in general and people also with Down syndrome, where as many as half of people with that condition develop dementia of some kind as they reach their 60s.
Andrea Pfeifer, AC Immune CEO and a co-author of the study, says in a company statement the findings are encouraging “because of the robust polyclonal immune response, including high concentrations of antibodies against highly neurotoxic pyroGlu-Abeta variants, which are thought to be key drivers of early AD. These neurotoxic pyroGlu-Abeta species are not strongly targeted by competing anti-Abeta vaccines.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on February 3rd, 2022  (Gerd Altmann, Pixabay. https://pixabay.com/illustrations/physics-quantum-physics-particles-3871218/) 3 Feb. 2022. Two companies formed in the last two years are applying quantum computing and artificial intelligence to discover new treatments for improving human longevity. Allosteric Bioscience in New York is taking an equity stake in Polaris Quantum Biotech or PolarisQB in Durham, North Carolina, but other financial details of the collaboration are not disclosed.
PolarisQB, formed in 2020, applies quantum computing and artificial intelligence to drug discovery. Quantum technology handles information in terms of probabilities, which describes infinite information components called quantum bits, or qubits, between values of 1 and 0. Conventional digital technologies, on the other hand, assign values of 1 or 0 to bits as the basis for nearly all of today’s systems. Quantum technology is based on quantum mechanics, a branch of physics for describing the behavior of matter and energy at atomic and sub-atomic levels.
This behavior varies widely from deterministic forms of physics, allowing for matter and energy to exist in multiple simultaneous states and interact while interconnected. Translated into information technologies, quantum properties open up opportunities to tackle difficult and complex challenges with simultaneous operations that would break most digital systems. Many of those challenges are complex scientific issues, such as discovering new drugs or addressing climate change.
Targets and modulators platforms
PolarisQB’s technology applies quantum computing and A.I. to drug discovery, for enacting multiple parallel searches of large-scale chemical libraries. The company says its processes make it possible to quickly assess interactions involving billions of molecules rather than many smaller evaluations linked together, as required by conventional computing. PolarisQB says by adding machine learning to the process, the company reduces the time needed for analytic and drug design computations even further.
Allosteric Bioscience, formed in July 2021, also uses quantum computing and A.I. for drug discovery, but applies the technology to the aging process. The company says its targets and modulators platforms focus on genetics, epigenetics, systems biology, and proteomics to identify processes affecting human longevity. Among the founders of Allosteric Bioscience is Peter Sordillo, chief scientist at SignPath Pharma and practicing oncologist in New York, who studied quantum information theory and quantum computers.
In the collaboration, the two companies are expected to apply quantum computing and A.I. to design an inhibitor of a key protein involved in the aging process. While responsibilities between the two companies are not spelled out, PolarisQB says it normally conducts initial synthesis and testing of new therapies, while licensing the treatments to pharmaceutical companies for further development.
Shahar Keinan, co-founder and CEO of PolarisQB, says in a company statement, “Quantum computing technology is coming of age, allowing us to revolutionize drug discovery timelines and budgets, while improving the overall profile of the designed drugs.” Keinan adds, “The application of quantum computers to solving these complex questions is truly extraordinary.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|










 RSS - Posts
RSS - Posts
You must be logged in to post a comment.